
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
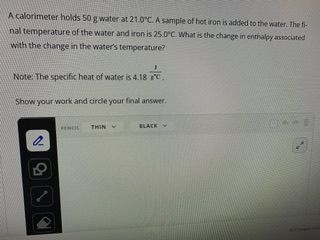
Transcribed Image Text:A calorimeter holds 50 g water at 21.0°C. A sample of hot iron is added to the water. The fi
nal temperature of the water and iron is 25.0°C. What is the change in enthalpy associated
with the change in the water's temperature?
Note: The specific heat of water is 4.18 °C.
18 FC.
Show your work and circle your final answer.
22
PENCIL THIN
BLACK
DE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A piece of metal weighing 146.7 g was heated from 32.0 °C to 56.2 °C. If the amount of heat needed was 1363.3 J, calculate the specific heat of the metal. What is the identity of the metal? 20 Specific Heats of Selected Metals Smetal, J/g-°C Metal 0.901 Smetal, J/g-°C 0.449 Metal Al Fe Cu 0.384 Ni 0.444 Si 0.711 Sn 0.226 Zn 0.389 Mn 1.02arrow_forwardIf 30.5 g of LiBr are dissolved 350.0 g of water at 20.0 °C in an insulated container, a temperature change is observed. The Δ H of solution of LiBr is -48.8 kJ/mol. Assuming that the specific heat of the solution is 4.184 J/(g C) and that no heat is gained or lost by the container, what will be the final temperature of the solution?arrow_forward(please type answer no write by hand)arrow_forward
- A chemist burned a sample of coal in a bomb calorimeter. Her results are below. Calorimeter's Heat Capacity 7.264 kJ/°C Mass of Coal Initial Temperature Final Temperature 0.100 g 22.07°C 25.37°C The sample of coal that the chemist placed in the bomb was dry but when she finished the test there was a small amount of water in the bomb. Where did this water come from and how does it affect her result? The water came from the water surrounding the bomb so her measured heat of combustion is for a closed system. The water was a product of combustion so her measured heat of combustion is for an open system. The water came from the water surrounding the bomb so her measured heat of combustion is for an open system. The water was a product of combustion so her measured heat of combustion is for a closed system.arrow_forwardA chemist heats the block of copper as shown in the interactive, then places the metal sample in a cup of oil at 25.00 °C instead of a cup of water. The temperature of the oil increases to 26.31 °C. Calculate the mass of oil in the cup. The specific heat of copper is 0.387 J/g °C and the specific heat of oil is 1.74 J/g. °C. moil = y TOOLSarrow_forward895/variants/431895/take/9/ TEXT ANSWER Question 10 A calorimeter holds 50 g water at 22.0°C. A sample of hot iron is added to the water. The final temperature of the water and iron is 28.0°C. What is the change in enthalpy associated with the change in the water's temperature? Note: The specific heat of water is 4.18 g°C Use the formula AH = - cm AT Show your work. Normal A A 3x S I = = = = 外 Iロび Enter your answer here Oo00 OO of 10 Total Questions Answered All Changes Saved Continue > 11:10 AM Vordsworth, Shelle.. OEdio | Course Stude. 书 5/5/2022arrow_forward
- CH4 (g) + 2 O2 (g) --> CO2 (g) + 2H2O (g) Δ H = -882 kJ/mol Using the equation above, how many grams of water will form if 655 kJ of energy was released.arrow_forward1) A 0.416 g sample of KCl is added to 56.5 g of water in a calorimeter. If the temperature decreases by 1.24°C, what is the approximate amount of heat (in J) involved in the dissolution of the KCl, assuming the heat capacity of the resulting solution is 4.18 J/g°C? _____ J 2) Is the reaction exothermic or endothermic?arrow_forward1. A thermometer placed in a solution undergoing a chemical reaction indicates an increase in temperature as the reaction proceeds. Is this reaction endothermic or exothermic? Describe if heat energy is lost or gained from the reaction (the system) to the surroundings. What is the sign of the enthalpy change (AH) of this reaction? 2. A student performs a reaction and determines the enthalpy change (AH) to be 31.4 kJ. Will the temperature of the surrounding solution increase or decrease as a result of this chemical process? 3. If you hold 3 grams of ice in your hand at room temperature, your hand will become cold. a) Is the reaction H,O(s) – H,O(1) endothermic or exothermic? b) In which direction does heat flow?arrow_forward
- Can you explain where did I go wrong, I keep getting the same answer.arrow_forwardSuppose a boil water notice is sent out advising all residents in the area to boil their water before drinking it or using it for cooking. You need to boil 18.0 L of water using your natural gas (primarily methane) stove. What volume of natural gas is needed to boil the water if only 10.1% of the heat generated goes towards heating the water. Assume the density of methane is 0.668 g/L, the density of water is 1.00 g/mL, and that the water has an initial temperature of 21.4 °C. Enthalpy of formation values can be found in this table. Assume that gaseous water is formed in the combustion of methane.arrow_forwardlp .html?ClassID=543616615# The calorimeter contains 900. g of water and the temperature change is 8.32 °C. The heat capacity of the calorimeter is 2240 J/°C. The specific heat of water is 4.18 J/g°C. 3: What is the heat of the calorimeter (water and dry combined)? 9cal = [ [? ] J Enter either a + or - sign AND the magnitude. Do not round. q.cal (J) Enterarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY