
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
can you please type this out and not write it, thank you!
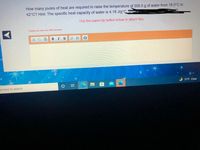
Transcribed Image Text:How many joules of heat are required to raise the temperature of 200.0 g of water from 18.0°C to
42°C? Hint: The specific heat capacity of water is 4.18 J/g°C. STOw=
Use the paperclip button below to attach files.
Student can enter max 3500 characters
I U
31°F Clear
pe here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Saved Normal BIIIU fxl Ix Actual mass used in solution prep (g) Volume solution prepared Measured conductivity (uS/cm) Substance 0.1 g Naci 0.1003 100 mL 1149 0.1 g Nal 0.10 100 ml 419 Calculate the mass of Nal that would be necessary to yield the same conductivity as the NaCl solution. Clearly show these calculations in your lab notebook. Saved T BII U X X + fr Normalarrow_forwardThe mystery fuel speedster! (Homework ) For fun, you build an explosive car with your little brother by attaching wheels to an empty windshield washer container. To advance it, you add 1.20 ml ( = 0.7915 g/ml) of a liquid but volatile fuel into the container. You close the cap and shake to evaporate the liquid. Finally, you place the car on its wheels, remove the cap and insert a burning wooden rod into the container. A flame suddenly comes out of the car, which is propelled with good speed in front of your little brother surprised, but all upset by the maneuver. The volatile fuel is composed of 37.5% carbon, 12.6% hydrogen and 49.9% oxygen. Its molar mass is approximately 32.0 g/mol. During the reaction, the fuel (gaseous) burns in the presence of gaseous oxygen, and the products of the reaction are carbon dioxide and water, gaseous too. The volume of the windshield washer container used is 4.00 litres. Useful Info: There is 21.0% gaseous oxygen in the air and initial conditions…arrow_forwarda W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 30.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 05 106 107 108 109 110 111 112 114 116 118 Ob Sg Bh Hs Mt Ds Uuu Uub 260) (263) (262) (265) (266) (271) (272) (277) (296) (298) (7) 60 61 62 63 64 65 66 67 68 69 70 71 Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 44.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 92 93 94 95 96 97 98 99 100 101 167.3 168.9 173.0 175.0 102 103 U Np Pu Am Cm Bk Cf F Es Fm Md No Lr 238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) sogna pee ou cacau pe busca o cus £(H၁)၁၀- шогеш аз моц моцѕ 01 смоле рәліп əsənу sәне иo pǝseq pod еш әсхә пол t be a part by a padd a test so be aarrow_forward
- There are 2 boxes for each reactant and product. Type the coefficient in the first box and the chemical formula in the second. Enter the chemical formulas in the order they appear in the problem. Each box must have a coefficient or formula. Type in a "1" if you did not change the coefficient. Type the formula in this format: CO_2. Omit the subscript "1" in the chemical formulas. This is case-sensitive. Carbon monoxide reacts with iron(III) oxide to produce iron and carbon dioxide.arrow_forwardThe chart shows average CO2 emissions for fossil fuel vehicles (gasoline, diesel, and hybrid gas‑electric), as well as electric vehicles. The ordinate is mg CO2 per mile driven per pound mass of the vehicle (called curb weight). For electric vehicles, CO2 emission varies in U.S. states because of the mix of fuels used to generate electricity. If electricity came entirely from solar power, there would be little CO2 associated with its production. California has the lowest CO2 emission for producing electricity and Ohio has the highest because it depends heavily on coal‑fired power plants. The chart does not include CO2 emission from manufacturing vehicles. When normalized for distance driven by each kind of vehicle before it is scrapped, CO2 emission associated with manufacturing electric vehicles is estimated to be 25–75% greater than CO2 emission associated with manufacturing gasoline and diesel vehicles. If manufacturing were included, CO2 emission associated with electric vehicles…arrow_forwardWhich of the following methods is not an appropriate method for purifying a solid under normal laboratory conditions? sublimation Ochromatography distillation recrystallizationarrow_forward
- 100% 41 Safari File Edit View History Bookmarks Window Help Wed 4:51 PM A uk.instructure.com My Questions | bartleby Course Details University of Kentucky - CHE 111 Lab - Fall19 - FRENCH: Quiz.. Calculate the Molarity of Sodium Hydroxide? | Yahoo Answers = CHE111-017-032 > CHE111 Sections 017 to 32 (Tuesdays): General Chemistry I Laboratory (Fall 2019) Fall 2019 Home Account Announcements Dashboard Question #: 11 Syllabus Course Details An aqueous solution containing 0.3845 g of KHP (KHC;H4O4) was titrated with a solution of sodium hydroxide, producing the following titration curve. What is the concentration of the NaOH solution? Courses Modules Titration of KHP with NaOH Piazza Groups 14 Grades 12 Calendar 10 Inbox 6. Help Volume NaOH (ml) DEC 18arrow_forward7. Write balanced equations for each of the following by inserting the correct coefficient in the blanks. OsH + 1DsM HOsM ZrO2(s) + HF() – ZFF4(s) + a. -> b. Na20(s) + H2O(1) → _ NaOH(aq) + O2(g) Al2O3(s) + SKCH COO →__ NaOH(aq) – NasAIO3(aq) + H2O(1) С.arrow_forwardOWLV2 | Online teaching and X 0mySigTau xb Login | bartleby X M COMM.1113: FUND OF ORA X G what food has c - Google Se X + om/ilrm/takeAssignment/takeCovalentActivity.do?locator=assignment-take D [References] hotogray lenses incorporate small amounts of silver chloride in the glass of the lens. When light hits the AgCl particles, the following reaction occurs: hv AgCl Ag + Cl The silver metal that is formed causes the lenses to darken. The enthalpy change for this reaction is 2.90 × 10² kJ/mol. Assuming all this energy must be supplied by light, what is the maximum vavelength of light that can cause this reaction? Wavelength %3D nm Submit Answer Try Another Version 3 item attempts remaining Previous Nextarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY