
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
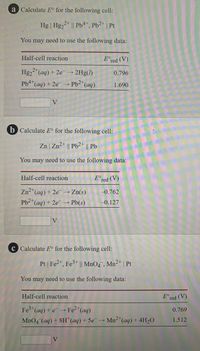
Transcribed Image Text:a Calculate E° for the following cell:
Hg | Hg22* || Pb4*, Pb2* | Pt
You may need to use the following data:
Half-cell reaction
E°red (V)
2+
Hg2 "(aq) + 2e 2Hg(1)
0.796
Pb4(ag) + 2e → Pb2*(aq)
1.690
V
b Calculate E° for the following cell:
Zn | Zn2* || Pb2+ || Pb
You may need to use the following data:
Half-cell reaction
E°red (V)
Zn-*(aq) + 2e → Zn(s)
-0.762
Pb2*(aq) + 2e → Pb(s)
-0.127
V
c Calculate E° for the following cell:
Pt | Fe2", Fe3* || MnO4 , Mn²* | Pt
You may need to use the following data:
Half-cell reaction
E°red (V)
Fe (aq) + e → Fe2*(aq)
MnO4 (aq) + 8H"(aq) + 5e→ Mn2"(aq) + 4H20
0.769
1.512
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A voltaic cell using Pb²⁺/Pb and Ni²⁺/Ni half-cells is set up at standard conditions, and each compartment has a volume of 355 mL. What is the cell potential, in V, after it has delivered 0.150 A for 51.0 hours at 25 °C? (E° for Pb²⁺/Pb = -0.130 V and E° for Ni²⁺/Ni = -0.250 V.)arrow_forward7-A fuel cell uses the following reduction half-reactions. O2(g) + 2H20(1) + 4e- 40H- (ag) E° = 0.401 V CO3-(aq) + 7H2O(1) + 8e– → CH«(g) + 100H-(ag) E° = 0.110 V Ja) What is the maximum cell voltage that can be generated (b) What is the overall cell reaction that occurs?arrow_forwardA voltaic cell prepared using aluminum and nickel has the following cell notation. Al(s) | AI3+(aq) || Ni2*(aq) | Ni(s) Which of the following reactions occurs at the cathode? Al(s) Al3*(ag) + 3e- O Ni2*(aq) + 2e- Ni(s) Al3+(aq) + 3e Al(s) > Ni(s) Ni2*(aq) + 2earrow_forward
- Consider the following voltaic cellat 25°C: Al(s) | Al3*(0.20 M, aq) || Al3+(0.75 M, aq) | Al(s) If the standard reduction potential for the Al3+(aq) /Al(s) redox couple is -1.66 V, what is the overall cell reaction and the cell potential of the voltaic cell shown above? (Reference the cell as written above.) The overall cell reaction is Al3+(0.75 M, aq) AIS*(0.20 M, ag) and E°cell = +0.0113 V. There is no overall cell reaction and E°cell = 0.00 V. The overall cell reaction is Al3+(0.20 M, aq) Als*(0.75 M, aq) and E°cell = +0.0113 V. The overall cell reaction is Al3+(0.20 M, aq) →A13+(0.75 M, ag) and E°cell| = -0.0113 V. The overall cell reaction is Al3*(0.75 M, aq) →AI3*(0.20 M, aq) and E°cell = -0.0113 V. None of thesearrow_forward3.) Consider the following standard reduction potentials, which statement is correct at standard conditions? Ni?* (aq) + 2 e → Ni (s) E°red = -0.26 V I2 (s) + 2 e -> 2 (aq) E°red = 0.54 V I. Ni²* (aq) is a stronger oxidizing agent than I2 (s), and I is a stronger reducing agent than Ni (s) II. I2 (s) is a stronger oxidizing agent than Ni2", and Ni (s) is a stronger reducing agent than I (aq) III. Ni (s) is a stronger oxidizing agent than I (aq), and Ni2* is a stronger reducing agent than I2 (s) IV. I(aq) is a stronger oxidizing agent than Ni (s), and I2 (s) is a stronger reducing agent than Ni2+ (aq) А. IV В. Ш С. П D. Iarrow_forwardPredict reaction and calculate E°cell from standard reduction potentials. A voltaic cell is constructed from a standard AI3+|Al half cell (E°red = -1.660V) and a standard F2|F° half cell (E°red = 2.870V). What is the spontaneous reaction that takes place, and what is the standard cell potential? Spontaneous cell reaction:Write a balanced equation using the smallest integer coefficients possible. + Standard cell potential: V.arrow_forward
- 5. What is voltage of the following cell? Sn(s) | Sn(NO3)2 (0.100 M) || AgNO3(0.020 M) | Ag(s)arrow_forwardPlease help with multiple choice questionarrow_forwardPlease complete the rollowing lab by answering questions 1-7. Draw your compounds clearly and indicate stereochemistry where appropriate. Feel free to consult others for help, including your instructor. 1.) For the following dienes, identify whether the molecules has any cis or trans stereochemistry and idenfity the conformation as either s-cis or s-trans, where appropriate. Please also note whether the compound would be reactive in a Diels-Alder reaction. H3C H3C. CH3 CH3 CH3 Br H3C Br 3)For the followng please draw osonco contrbutors 2.) en cile ntribut abybnd resonance id a.) Devise a retrosynthetic analysis for the synthesis of the molecule shown below. Be sure to draw all starting materials and conditions. CH3 CH3 1arrow_forward
- A voltaic cell is constructed with two silver electrodes, where the half-reaction isAg2+(aq) + 2e- → Ag (s) E° = -0.322 VDetermine the cell emf at 25 °C when the concentrations of chloride ion in the two compartments are 4.0 Mand 0.050 M, respectively.arrow_forwardA lithium battery is being developed. Its voltaic cell is constructed based on Li metal electrode and PbsO4 solid electrode. Their standard reduction potentials at 25 °C are given below. E°(Li* (aq) → Li (s)) = -3.040 Volt E°(PbSO4 (S) > Pb (s) + SO42 (aq) = - 0.356 Volt. Which of the following statements is true about this working (spontaneous) voltaic cell? a. The anode is PbsO4 (s) electrode. The standard cell potential of this voltaic cell is E°(cell) = 2.328 Volt O. PBSO4 (s) is a weaker oxidizing agent than Lit. To make a commercial product, the voltaic cell is adjusted to have [Lit] = 2.1 M and [SO42] = 3.5 M. The cell d. potential E under this condition should be less than the standard cell potential E°(cell). e. None of the abovearrow_forwardhalf-reaction standard reduction potential Cl,(9)+2e e¯ → 2ci^(aq) E =+1.359 V (red 0,(9)+4H"(aq)+4e¯ → 2 H,0(1) - 2 =+1.23 V red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have Yes enough information to calculate the cell voltage under standard conditions? No If you said it was possible to calculate the cell voltage, do so and enter your answe here. Round your Ov answer to2 sianificant diaits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY