
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:HW-1 Spring 2022
w NWP Assessment Player UI Appli x
Bb My Blackboard Content- Blackb x
Ô https://education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=6f2e7158-9fb3-4cf3-9a59-ac1e53c5..
022
Question 13 of 15
0/0.5
View Policies
Show Attempt History
Current Attempt in Progress
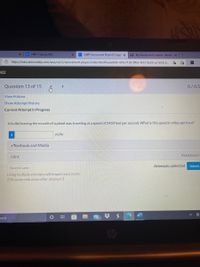
A bullet leaving the muzzle of a pistol was traveling at a speed of 2450 feet per second. What is this speed in miles per hour?
i
mi/hr
eTextbook and Media
Assistance
Hint
Attempts: unlimited
Submit.
Save for Later
Using multiple attempts will impact your score.
25% score reduction after attempt 3
arch
[99+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- er| Portal x Edulastic O Science Spring Semester Exm bapp.edulastic.com/student/assessment/5f18467bc420a100086da 115/cla 28b60984e66etd62fac10/uta/6a9 Elementar. K! Kahoot R ReadWorks Play Quizizz! Newsela Assignm Commontty As ty Play no. Question 5/10 > NEXT BOOKMARK Water is boiled and steam is produced. This is a physical change because the water particles A. have turned into something new. B. are still water particles. C. have reacted with each other. D. have been destroyed. B.arrow_forwardmid W Conte WP Quest X A Player x A Player x Ether X A Player X we Wiley X 13.5 PX 913.16 X Ama: x ▸ Spect x W x G Goog x C education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=52f59e64-c3d2-496f-9173-d76be854920c#/question/20 ☆ 01 YouTube Maps A LASC459001.20231... A Do It Later Meeting | Microsoft... spanish while you sl... NC FAST Learning G... 19 Creative Ideas fo... Theme of the Week... 100 Summer Camp apter 13 - Homework 2 Question 21 of 40 For the following reaction, predict the major product and draw a mechanism for its formation. 1) LIAIH Me 2) H₂O ? 13.16d Part 1 Incorrect. Modify the given structure of the starting material to draw the major product." H H Me Edit Drawing 0/1 E : eTextbook and Media Assistance Used Q Search mshparrow_forwardersion xxam e... /myct/itemView?assignmentProblemID=191818234 ent the basis ween and e of or S d ror f5 % 5 X ▸ Part A Final Exam America.... TN Free Tracing Letter... Letter A | Workshee... Submit Part B Part C 16 Solving Chemical Problems G| ΑΣΦ 3 10 A In Europe, gasoline efficiency is measured in km/L. If your car's gas mileage is 28.0 mi/gal, how many liters of gasoline would you need to buy to complete a 142-km trip in Europe? Use the following conversions: 1 km = 0.6214 mi and 1 gal = 3.78] Express your answer numerically in liters. ► View Available Hint(s) 6 4- & C hp 7 X f8 a + MasteringChemistry: Homework x * ? 8 L Order 3094227- Es... fg 9 h fio ) fi O Review | Constants | Periodic Table f12 + O 6 of 10 ins = M 1:13 PM 8/16/2022 prt sc > del backsarrow_forward
- 13 ← CO Biblio Manuels-fren.... CH Draw the curved arrows showing a proton transfer reaction, and draw the products of that proton transfer. Do not include the Li+ counterion, and lone pairs are not required in the products. + H-O-H Edit Drawing CMCbx bbwb + C .../1 11:11 PM 2024-02-09 0 AINarrow_forwardCHRIRAD mework WP NWP Assessment Player Ul Appli X tion.wiley.com/was/ui/v2/assessment-player/index.html?launchld=d7fbf299-2b78-4aab-9fe4-25d65ce73435#/question/5 Question 6 of 6 W Carbon tetrachloride (CC14) was prepared by reacting 119 g of carbon disulfide and 119 g of chlorine. Calculate the percent yield if 65.0 g of CCl4 were obtained from the reaction CS₂+3 Cl₂ → CCl4 + S₂Cl2. Save for Later + % yield Want to see a relevant text example? GO Tutorial -/2 = O Search کا Attempts: 0 of 5 used Submit Answerarrow_forwardM Inbox (321) - jjuhasz@ X in Course: NTR-3230-130 X My Courses Course Home Syllabus Scores Pearson eText Study Area Document Sharing User Settings Course Tools @ EH ✰ openvellum.ecollege.com/course.html?courseld=17762365&OpenVellumHMAC=57891f531d784362508555aa651bb397#10001 > e X Course Homearrow_forwardNWP Assessment Player UI Appli X WP -> A https://education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=eb66b5b0-1212-4354-98f4-985dc387b4b3... В NEW E Apps P Pearson Sign In Sign in to your acco... E Set 04 - SP22 Question 11 of 23 - / 1 Draw an organobromide that will react with lithium diphenylcuprate to give the following compound: OCH3 Draw Your Solution 10:48 PM O Type here to search 30°F a ») ENG 1/29/2022arrow_forwardNWP Assessment Player UI Appli X WP -> A https://education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=eb66b5b0-1212-4354-98f4-985dc387b4b3... В NEW E Apps P Pearson Sign In Sign in to your acco... E Set 04 - SP22 Question 5 of 23 1/5 Add curved arrow(s) to draw step 1 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step (do not draw the counterion). Be sure to include relevant stereochemistry at all chiral centers by using the dashed and wedged bond tools to draw new bonds (the direction of these bonds can be changed by clicking on a drawn bond). H,ö=-CH3 + CH3 Et-Mg-Br CI A Edit Drawing 10:47 PM O Type here to search 30°F 1/29/2022arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help 3 Ch 7: Homework: Viridiana Ag x O NWP Assessment Player UI A X ? 92% - Wed 10:19 PM Q i assessment.education.wiley.com/was/ui/v2/assessment-player/index.html?launchid-98198bfd-3a6f-42ed-9bcc-6cec951a6966#/question/8 + Ch 7: Homework Question 9 of 10 0.5/1 View Policies Show Attempt History Current Attempt in Progress Your answer is partially correct. Copper is often added to paint for ocean-going ships and boats to discourage the growth of marine organisms on their hulls. Unfortunately, the copper dissolves into the water and is toxic to the marine life in harbors where these boats are docked. When copper(II) ions reach a concentration of 9.0 micrograms/liter, the growth of some marine life slows. How many copper ions are found in a liter of water containing 6.9 micrograms of Cu2* per liter? i 4.45 x 10 16 Cu2* ions eTextbook and Media Hint Attempts: 1 of 5 used Submit Answer Save for Later F9 F7 F5 F4 F2 * & 23 $ 8 9 @ 4…arrow_forwardConte X ALEK X O Rich x Ceng Bb Excel Bb What X O COVI e Daily x O Amon x e Chat N Ne www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsikr7j8P3jH-lIJOkWvnm4w-aQ-rw-zRhgRnayfmbBs65spEVIGu-K5Jx48w9eOKISqsha Click to go forward, hold to see history Apps R Blackboard 2Mail - Ava Schied. O UAConnect Biology Syllabus J Labflow - Courses O Explore - HogSync O Packback + MyN Objective Knowledge Check Question 13 Calculate the mass of glucose (C,H,,0,) that contains a trillion (1.0 x 102) oxygen atoms. Be sure your answer has a unit symbol if necessary, and round it to 2 significant digits. I Don't Know Submit O 2020 McGraw-Hill Edu MacBook Droarrow_forward) Statistics (AA) - Live Online O Course Home C If Solar Radiation Is 1140 W/m2 X O Study.com - Search for courses, x O 8 https://openvellum.ecollege.com/course.html?courseld= 16914186&OpenVellumHMAC=c662ec09ac8023bd 1b971c039afd0082#10001 View Available Hint(s) E = kJ/mol Submit Previous Answers X Incorrect; Try Again; 4 attempts remaining Part B Y-ray photons with a wavelength of 2.54x10-5 nm Express the energy numerically in kilojoules per mole. View Available Hint(s) here to search L 52% Home End Insert Delete %23 2$ 4 6 7 8 Backspace Y D F G H J KL Enter C VB NM 5arrow_forward一 一 HW-1 Spring 2022 wP NWP Assessment Player Ul Appli X Bb My Blackboard Content- Blackb X + https://education.wiley.com/was/ui/v2/assessment-player/index.html?launchld%3D6f2e7158-9fb3-4cf3-9a59-acle53c5... Question 15 of 15 -/ 1 iew Policies Current Attempt in Progress The brightest star in the night sky in the northern hemisphere is Sirius. Its distance from Earth is estimated to be 8.7 light years. A light year is the distance light travels in one year. Light travels at a speed of 3.00 x 10° m/s. Calculate the distance from Earth to Sirius in miles. (1 mi =5280 ft). x 10 eTextbook and Media Hint Attempts: unlimited Submit Answer Save for Later Using multiple attempts will impact your score. 25% score reduction after attempt 3 search +66) fe 144 16 10 00arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY