
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A general chemistry student found a chunk of metal in the basement of a friend's house.To figure out what it was,she used the ideas just developed in class about density.
She measured the mass of the metal to be 111.2 grams.Then she dropped the metal into a measuring cup and found that it displaced 15.2mL of water.
Calculate the density of the metal.
Density= G/mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Dilution A chemist must dilute 22.0 mL of 45.9 μM aqueous magnesium fluoride (MgF₂) solution until the concentration falls to 39.0 μM. She'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits. mL X 3arrow_forwardA student is determining the density of an unknown metal with a mass of 87.8 g. The student partially fills a graduated cylinder with water and measures the volume of the water by itself as 54.8 mL. The student then adds the metal to the water and measures the new volume as 87.3 mL. What is the density, in g/mL, of the metal?arrow_forwardA chemist mixes two liquids, A and B, to form a homogeneous mixture. The densities of the liquids are 2.0514 g/mL for A and 2.6678 g/mL for B. When she drops a small object into the mixture, she finds that the object becomes suspended in the liquid; that is, it neither sinks nor floats. If the mixture is made of 41.37 percent A and 58.63 percent B by volume, what is the density of the metal? Group of answer choices 2.413 g/mL 1.753 g/mL 2.691 g/mL 2.015 g/mL 1.913 g/mLarrow_forward
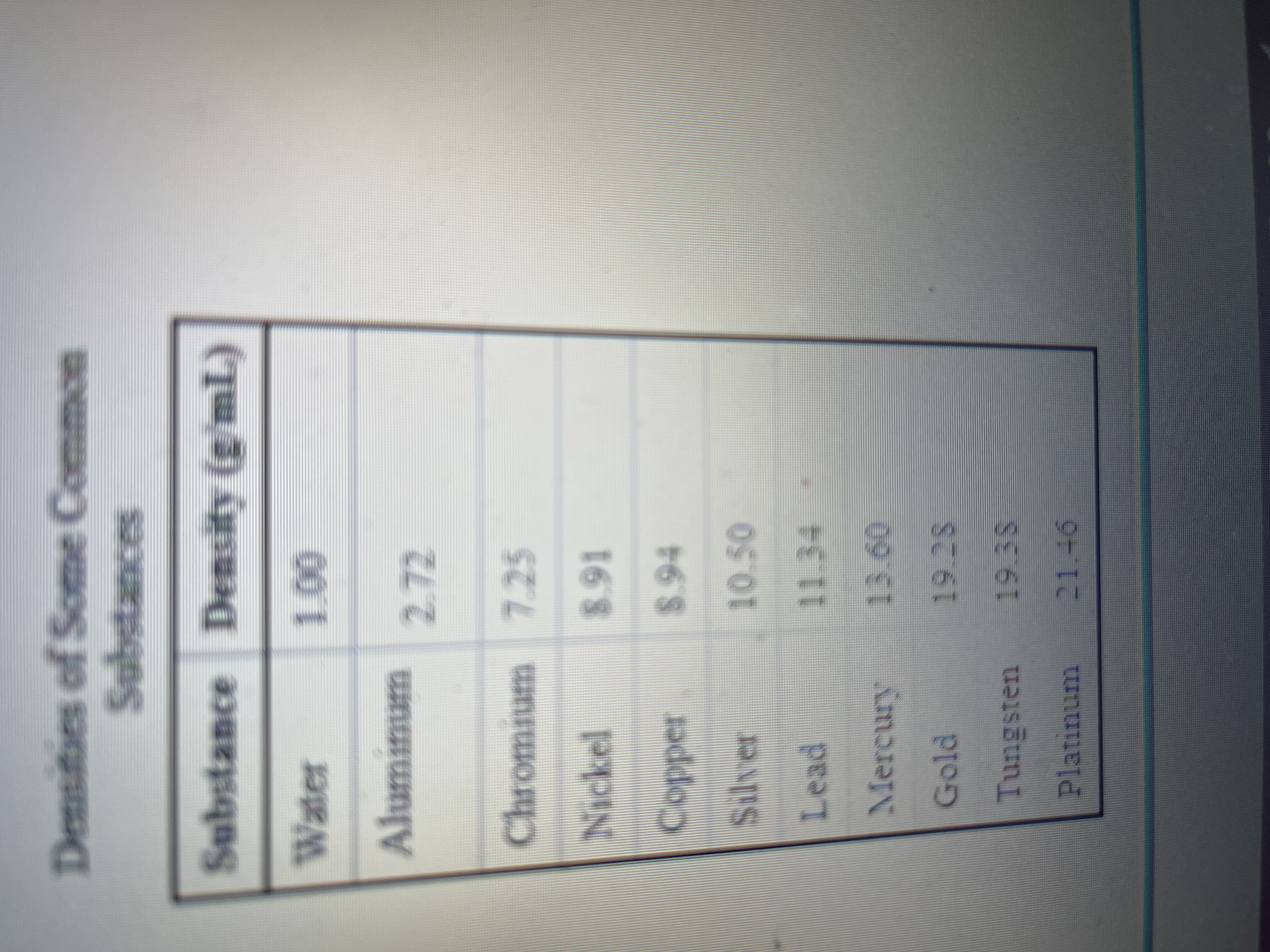
- A student wishes to determine the density of a solution of lithium hydroxide. He finds the mass of the flask to be 27.532 g. When he fills the flask with water at 22℃ (d= 1.00 g/mL), its mass is 37.441 g. He finds that the mass of the flask filled with lithium hydroxide solution is 38.138 g. What is the density of the lithium hydroxide solution?arrow_forwarda student has been given a solid sample and asked to calculate its density. She measured the mass of the solid sample and recorded it on her data sheet as 4.528g. She recorded the initial volume as 4.7 mL and final volume as 6.2 mL. What is the density of the samplearrow_forwardUse the References to access important values if needed for this question. A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she used the ideas just developed in class about density. She measured the mass of the metal to be 323.0 grams. Then she dropped the metal into a measuring cup and found that it displaced 16.5 mL of water. Calculate the density of the metal. Density= g/mL Densities of Some Common Substances Substance Density (g/mL) Water Aluminum 2.72 Chromium 7.25 Nickel Copper Silver Lead Mercury 1.00 Gold Tungsten Platinum 8.91 8.94 10.50 11.34 13.60 19.28 19.38 21.46 Previous Next> ?arrow_forward
- A pycnometer is a glass apparatus used for accurately determining the density of a liquid. When dry and empty, a certain pycnometer had a mass of 27.234 g. When filled with distilled water at 25.0°C, it weighed 36.491 g. When filled with chloroform (a liquid once used as an anesthetic before its toxic properties were known), the apparatus weighed 40.946 g. At 25.0°C, the density of water is 0.99704 g/mL (a) What is the volume of the pycnometer? i mL (b) What is the density of chloroform? i ! g/mL eTextbook and Media Attempts: 2 of 15 used Submit Answer Save for Later 山T 19 MacBook Air DII DD 吕0 888 F8 F10 F5 F6 F7 F3 F4 F1 F2 #3 $ % & 2 3 5 7 8.arrow_forwardA general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she used the ideas just developed in class about density. First she measured the mass of the metal to be 198.1 grams. Then she dropped the metal into a measuring cup and found that it displaced 17.3 mL of water. Calculate the density of the metal.arrow_forwardThe density of methanol, a colorless organic liquid used as solvent, is 0.7918 g/mL. Calculate the mass of 89.9 mL of the liquid.arrow_forward
- please make sure the answer has the correct number of significant digitsarrow_forwardBud N. Chemist must determine the density of a mineral sample. His four trials yield densities of 5.27 g/cm3, 5.28 g/cm3, 5.27 g/cm3, and 5.29 g/cm3. Bud's calculated average density was 5.28 g/cm3. Independent studies found the correct density to be 4.75 g/cm3. Which of the following statements represents the best analysis of the data? Bud's results have much greater accuracy than precision Bud's results have low accuracy and low precision Bud's results have high accuracy and high precision Bud's results have much greater precision than accuracyarrow_forwardAn iceberg has a volume of 8365 ft^3 What is the mass in kilograms of the iceberg? The density of ice in the iceberg is 0.92 g/cm^3. Express your answer using two significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY