
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
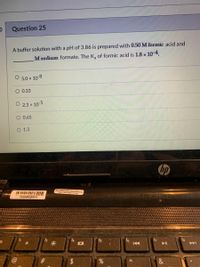
Transcribed Image Text:Question 25
A buffer solution with a pH of 3.86 is prepared with 0.50 M formic acid and
M sodium formate. The K, of formic acid is 1.8 x 10-4.
5.0 x 10-8
O 0.33
O 2.3 x 10-5
O 0.65
O 1.3
hp
Nengli Te Don
732006020610
米
fs
&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the Ka of propionic acid if a buffer contains 0.150 M C3H6O2 and 0.100 M NaC3H5O2, and the pH is 4.71?arrow_forwardConsider a buffer solution contains 0.20 M NaF and 0.30M HF:- (K (HF) = 5.0 x 10-4 What is the pH of this solution? Choose... Calculate the pH after 0.025 mol of NaOH is added to the buffer Choose... Calculate the pH after 0.02 mol of HCl is added to the buffer Choose... Which of the following is NOT an acid-conjugate base pair? O NaOH/ Naz0arrow_forwardA 34.8 mL aliquot of weak acid that has a concentration of 0.752 M will be titrated with 0.853 M NaOH. Calculate the pH of of the solution upon the addition of 0 mL of HCl. The Ka of the acid is 7.5×10-6. A 36.54 mL aliquot of weak acid that has a concentration of 0.255 M will be titrated with 0.981 M NaOH. Calculate the pH of of the solution upon the addition of 40.11 mL of NaOH. The Kb of the base is 7.9 × 10-7.arrow_forward
- 33)arrow_forward4. What is the pH of a buffer made from 0.42 M H3BO3 and 0.22 M H2BO3-, if the Ka for H3BO3 is 7.3 x 10-10 ? (use 2 decimal places for pH)arrow_forwardWhat ratio of base to acid is needed to make a buffer with formic acid, HCOOH, and potassium formate, KHCOO buffer with pH = 3.76? Enter the ratio as a decimalarrow_forward
- An buffer is prepared by combining 4 L of a 0.57 M X solution with 4 L of an 0.57 molar HXCI solution. The K, of X is 4.54 ×10-3. What is the pH of this buffer solution? Please express your answer to the second decimal place.arrow_forwardO ACIDS AND BASES Calculating the pH of a weak base titrated with a strong acid with a 0.3000 M solution of HNO,. The p K, of An analytical chemist is titrating 160.2 mL of a 0.5000 M solution of dimethylamine ((CH₂)2NH) w dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 280.8 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places... PH-0 Xarrow_forwardA buffer solution with a pH of...arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY