
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I need help...
Transcribed Image Text:d
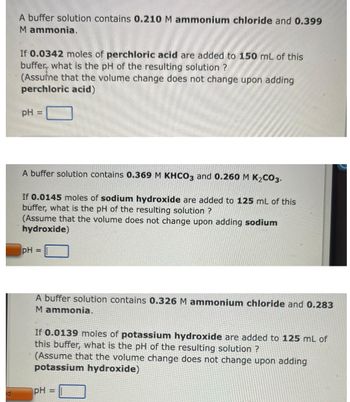
A buffer solution contains 0.210 M ammonium chloride and 0.399
M ammonia.
If 0.0342 moles of perchloric acid are added to 150 mL of this
buffer, what is the pH of the resulting solution ?
(Assume that the volume change does not change upon adding
perchloric acid)
pH
=
A buffer solution contains 0.369 M KHCO3 and 0.260 M K₂CO3.
If 0.0145 moles of sodium hydroxide are added to 125 mL of this
buffer, what is the pH of the resulting solution ?
(Assume that the volume does not change upon adding sodium
hydroxide)
pH
=
A buffer solution contains 0.326 M ammonium chloride and 0.283
M ammonia.
If 0.0139 moles of potassium hydroxide are added to 125 mL of
this buffer, what is the pH of the resulting solution ?
(Assume that the volume change does not change upon adding
potassium hydroxide)
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- part d please!!arrow_forwardThe main issues with coral reefs in great barrier Australia.arrow_forwardInbo (534) Conv I Balar b Ansv Post CHE 101 C X The bartl bartl bartl Cher Unkr O Sear E I ma G what app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 31 of 40 Submit A 35.0 mL solution of NaOH is neutralized with 26.5 mL of 0.250 M HCI. What is the concentration of the original NaOH solution? | M 1 4 C 7 8 9 +/- х 100 + 11:15 PM e Type here to search 59°F 8/26/2021 LO (8)arrow_forward
- doug began preparing laboratory surface disinfectant from chlorine bleach. he put on a chemical resistant apron and gloves and then removed the bleach container from the special chemical cabinet. he carefully placed the container on the laboratory benchtop and began to add the chlorine bleach to distilled water. nearby workers began complaining of burning eyes. doug was reprimanded by the supervisor. Explain why.arrow_forward1. Which of the following is not a step in preparing a water sample container?a. All sample containers must be dark in colorb. The type of sample container and the level of cleaning required depend on the type of sample to be takenc. All sample containers must be thoroughly cleaned in the laboratory before sampling is carried outd. The number of containers prepared must always be in excess of what is needed, for quality assurance, quality control and reserves 2. The purpose of environmental sample analysis is..a. To determine the origin and concentration of chemicals in the environmentb. To determine the origin, concentration of chemicals and/or pollutants in the environmentc. To determine the concentration of a chemical in the environmentd. To determine the cause and concentration of pollutants in the environmentarrow_forwardInbo (534) Conv I Balar b Ansv Post CHE 101 C X с Chec bartl bartl The b My C Unkr O Sear E I ma G what app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 28 of 40 Submit The OH concentration in an aqueous solution at 25 °C is 6.1 x 10 5. What is [H*]? 1 4 C 7 8 9 +/- х 100 + 10:59 PM e Type here to search 59°F 8/26/2021 LO (8)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY