
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
Answer asap
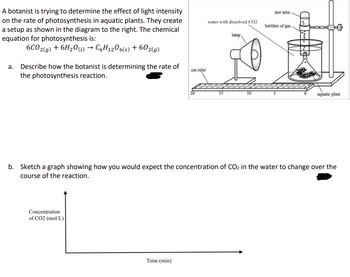
Transcribed Image Text:A botanist is trying to determine the effect of light intensity
on the rate of photosynthesis in aquatic plants. They create
a setup as shown in the diagram to the right. The chemical
equation for photosynthesis is:
6CO2(g) + 6H2O (l) → C6H12O6(s) + 602(g)
a. Describe how the botanist is determining the rate of
the photosynthesis reaction.
cm ruler
test tube
water with dissolved CO2
bubbles of gas
lamp
0
10
S
aquatic plant
b. Sketch a graph showing how you would expect the concentration of CO2 in the water to change over the
course of the reaction.
Concentration
of CO2 (mol/L)
Time (min)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The label on a bottle of 3% (by volume) hydrogen peroxide, H2O2, purchased at a grocery store, states that the solution should be stored in a cool, dark place. H2O2decomposes slowly over time, and the rate of decomposition increases with an increase in temperature and in the presence of light. However, the rate of decomposition increases dramatically if a small amount of powdered MnO- is added to the solution. The decomposition products are H2O and O2. MnO2 is not consumed in the reaction. Write the equation for the decomposition of H2O2. What role does MnO2 play? In the chemistry lab, a student substituted a chunk of MnO2 for the powdered compound. The reaction rate was not appreciably increased. WTiat is one possible explanation for this observation? Is MnO2 part of the stoichiometry of the decomposition of H2O2?arrow_forwardSilicon forms a series of compounds analogous to the al-kanes and having the general formula SinH2n+2. The first of these compounds is silane, SiH4, which is used in the electronics industry to produce thin ultrapure silicon films. SiH4(g) is somewhat difficult to work with because it is py-ropboric at room temperature—meaning that it bursts into flame spontaneously when exposed to air. (a) Write an equation for the combustion of SiH4(g). (The reaction is analogous to hydrocarbon combustion, and SiO2 is a solid under standard conditions. Assume the water produced will be a gas.) (b) Use the data from Appendix E to calculate ? for this reaction. (c) Calculate G and show that the reaction is spontaneous at 25°C. (d) Compare G for this reaction to the combustion of methane. (See the previous problem.) Are the reactions in these two exercises enthalpy or entropy driven? Explain.arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forward
- Iodomethane (CH3I) is a commonly used reagent in organic chemistry. When used properly, this reagent allows chemists to introduce methyl groups in many different useful applications. The chemical does pose a risk as a carcinogen, possibly owing to iodomethanes ability to react with portions of the DNA strand (if they were to come in contact). Consider the following hypothetical initial rates data: [DNA]0 ( mol/L) [CH3I]0 ( mol/L) Initial Rate (mol/Ls) 0.100 0.100 3.20 104 0.100 0.200 6.40 104 0.200 0.200 1.28 103 Which of the following could be a possible mechanism to explain the initial rate data? MechanismIDNA+CH3IDNACH3++IMechanismIICH3ICH3++ISlowDNA+CH3+DNACH3+Fastarrow_forwardDefine stability from both a kinetic and thermodynamic perspective. Give examples to show the differences in these concepts.arrow_forwardThe reaction NO(g) + O,(g) — NO,(g) + 0(g) plays a role in the formation of nitrogen dioxide in automobile engines. Suppose that a series of experiments measured the rate of this reaction at 500 K and produced the following data; [NO] (mol L ’) [OJ (mol L 1) Rate = -A[NO]/Af (mol L_1 s-1) 0.002 0.005 8.0 X 10"'7 0.002 0.010 1.6 X 10-'6 0.006 0.005 2.4 X IO-'6 Derive a rate law for the reaction and determine the value of the rate constant.arrow_forward
- Candle wax is a mixture of hydrocarbons. In the reaction of oxygen with candle w ax in Figure 11.2, the rate of consumption of oxygen decreased with time after the flask was covered, and eventually' the flame went out. From the perspective of the kinetic-molecular theory, describe what is happening in the flask. FIGURE 11.2 When a candle burns in a closed container, the flame will diminish and eventually go out. As the amount of oxygen present decreases, the rate of combustion will also decrease. Eventually, the rate of combustion is no longer sufficient to sustain the flame even though there is still some oxygen present in the vessel.arrow_forwardSome bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an enzyme with a molecular weight of 3104 g/mol that converts penicillin into inactive molecules. Although the kinetics of enzyme-catalyzed reactions can be complex, at low concentrations this reaction can be described by a rate equation that is first order in the catalyst (penicillinase) and that also involves the concentration of penicillin. From the following data: 1.0 L of a solution containing 0.15 g ( 0.15106 g) of penicillinase, determine the order of the reaction with respect to penicillin and the value of the rate constant. [Penicillin] (M) Rate (mol/L/min) 2.0106 1.01010 3.0106 1.51010 4.0106 2.01010arrow_forwardOne mechanism for the destruction of ozone in the upper atmosphere is a. Which species is a catalyst? b. Which species is an intermediate? c. Ea for the uncatalyzed reaction O3(g)+O(g)2O2(g) is 14.0 kJ. Ea. for the same reaction when catalyzed is 11.9 kJ. What is the ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction at 25C? Assume that the frequency factor A is the same for each reaction.arrow_forward
- The reaction for the Haber process, the industrial production of ammonia, is N2(g)+3H2(g)2NH3(g) Assume that under certain laboratory conditions ammonia is produced at the rate of 6.29 ×10-5 molL-1s-1. At what rate is nitrogen consumed? At what rate is hydrogen consumed?arrow_forwardConsider the reaction of ozone and nitrogen monoxide to form nitrogen dioxide and oxygen. O3(g) + NO(g) NO2(g) + O2(g) Which of the following orientations for the collision between ozone and nitrogen monoxide could perhaps lead to an effective collision between the molecules? (a) (b) (c) (d)arrow_forwardThe following rate constants were obtained in an experiment in which the decomposition of gaseous N2O; was studied as a function of temperature. The products were NO, and NO,. Temperature (K) 3.5 x 10_i 298 2.2 x 10"4 308 6.8 X IO-4 318 3.1 x 10 1 328 Determine Etfor this reaction in kj/mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning