
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:A book sitting on the top shelf of a bookcase has a lot of stored kinetic energy.
True
False
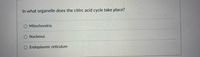
Transcribed Image Text:In what organelle does the citirc acid cycle take place?
Mitochondria
O Nucleous
O Endoplasmic reticulum
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- To reach the native state, the unfolded state has to go through a _____________ state which is more energetically unstable than both the unfolded and the native states.arrow_forwardPlease answer this. Type the answers out, please don't handwrite it I can barely read it that way. Thank youarrow_forwarduce the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 132. °C. AH is (pick one) AS is (pick one) His (pick one) B This reaction is endothermic. AS is (pick one) This reaction is spontaneous except below 110. °C but proceeds at a slower rate below 135. °C. AH IS (pick one) AS is (pick one)varrow_forward
- Fill in blank: An ES complex that releases 30 kj/mol of binding free energy will _____ (raise or lower) the activation energy barrier needed to reach the transition state.arrow_forwardWhat these letters represent? This picture is the effect of the energy graph.arrow_forwardPart A For the reaction Br2 (1) 2 Br (g) at what temperature is the equilbrium constant equal to 3.5? t> VO ΤΙ ΑΣΦ 0 ? Your answer should not contain commas. No credit lost. Try again. Submit Previous Answers Request Answer Karrow_forward
- Question 43 Which of the following is NOT a property of water? O exhibit cohesion O Ice is less dense than water water molecules are polar water molecules require a small input of energy to raise the temperature O high heat of vaporization Question 44 The first law of Thermodynamics state that: O energy must come in the form of a three-prong outlet the ultimate source of energy in our universe is the sun energy can be created and destroyed in a vacuum O energy can neither be created nor destroyedarrow_forwardatoms. 12 hydrogens, 6 oxuger Click or tap here to enter text.C atoms. 12) Back to the person who ate 300 grams of glucose, how many moles of glucose is this? Click or tap here to enter text. There are 2 moles. 13) If a person were to eat this much glucose all at once (that is a lot of sugar) and all this sugar enters the blood then the person's blood sugar concentration would rise. Imagine that all this glucose is released into the 5 liters of blood that circulate in this person. What would be the molarity of glucose in the blood? Click or tap here to enter text. 14) After each of these glucose molecules enter the blood they are uptaken through cell membranes to enter cells. Within these cells glucose is broken down in a series of steps releasing carbon dioxide along the way. For every molecule of glucose broken down how many molecules of carbon dioxide are created? Refer to the cellular respiration equation: Equation for Cellular Respiration: How to break down a carb C6H12O6 + 60₂ ➜…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education