
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
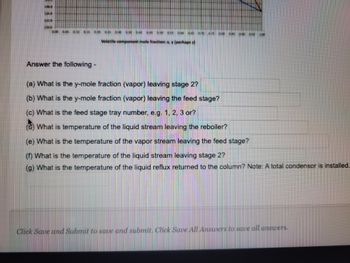
A binary distillation is performed so that a distillate 94% and bottoms 8% in of the more volatile Component A results. The process shown below reflects one operating condition of a feed stream.
Rectification, Stripping and the q-line are all provided on the x-y diagram below. Aling with expected stage for the distillation..
Solve a), b) c)
Transcribed Image Text:1M
1348
1.23
LINU
0.00 0.05 0.10 8.15
0.30 0.250.300.350.400.450.50 855 64 165 5 *
Volatile component mole fraction: x, y (perhaps x)
** 090 6.95
O
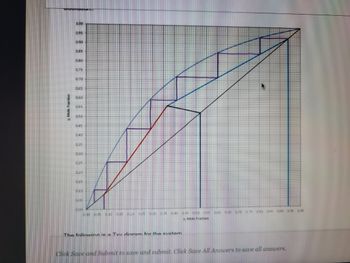
Answer the following -
(a) What is the y-mole fraction (vapor) leaving stage 27
(b) What is the y-mole fraction (vapor) leaving the feed stage?
(c) What is the feed stage tray number, e.g. 1, 2, 3 or?
What is temperature of the liquid stream leaving the reboiler?
(e) What is the temperature of the vapor stream leaving the feed stage?
(f) What is the temperature of the liquid stream leaving stage 2?
(g) What is the temperature of the liquid reflux returned to the column? Note: A total condensor is installed.
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Transcribed Image Text:andrammaron
y, Mole Fraction
1.00
0.95
0.90
085
0.80
0.75
0.70
065
0.60
0.55
0.50
0.45
0.40
0.35
0.30
0.75
0.20
015
010
DOS
DOD
0.00 0.05 0.10 0.15 0.20 3.25 0.30 0.35 0.40 0.45 0.500.55
. Mole Fraction
The following is a Tvy dinram for the custom
0.90 0.95 1.00
Sa 0.85
INING X 68 DU 0.75
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- GIVE A SIMPLE CALCULATION ON HOW TO GET TO 300LA first order irreversible reaction is conducted in an 800 L continuously stirred tank, in liquidsolution at 80 ᵒC temperature. The total conversion in the tank is calculated to be 60%. However,this conversion appears low.After a quick check, you realise that something in the mixing is not working correctly and as aresult, the tank is not optimally stirred.Following a few tests on residence times, you calculate that approximately 300 L of the tank do notinteract appreciably with the input or output streams. You therefore decide to modify the reactorby improving the stirring performance in order to obtain complete mixing in the reactor volume.arrow_forwardHelp with the following questionarrow_forwardA binary distillation is performed so that a distillate 94% and bottoms 8% in of the more volatile Component A results. The process shown below reflects one operating condition of a feed stream. Rectification, Stripping and the q-line are all provided on the x-y diagram below. Aling with expected stage for the distillation.. What is temperature of the liquid stream leaving the reboiler? What is the temperature of the vapor stream leaving the feed stage? What is the temperature of the liquid stream leaving stage 2? What is the temperature of the liquid reflux returned to the column? Note: A total condensor is installed.arrow_forward
- DRAW 2D DIAGRAM OF A SOLUTION TO MAKE THE MIXING MUCH MORE EFFICIENT A first order irreversible reaction is conducted in an 800 L continuously stirred tank, in liquid solution at 80 ᵒC temperature. The total conversion in the tank is calculated to be 60%. However, this conversion appears low.After a quick check, you realise that something in the mixing is not working correctly and as a result, the tank is not optimally stirred. Following a few tests on residence times, you calculate that approximately 300 L of the tank do not interact appreciably with the input or output streams. You therefore decide to modify the reactor by improving the stirring performance in order to obtain complete mixing in the reactor volume. Describe what considerations allowed you to evaluate the ‘unused’ volume in the tank and defend your proposed solution in a brief technical report to be presented to your manager for approval. The report must include a clear schematic drawing of your selected solutionarrow_forwardPlease answerarrow_forwardWrite a technical report in no more than five pages on Potash processing using hot leach process and cold crystallization process as: 1- Describe the impact of the following on the hot leach process: a. solar pans, mother liquor loop, how does crystallization of KCI occur in this plant and what happens to the pressure in these crystallizers. 2- Describe the technical operations in each step of the cold crystallization. 3- Compare both processes in terms advantages and disadvantages.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The