
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A base runner slides into the base. During this process, the energy transformation is _______. (Hint: Figure 10.2)
|
K to Ug |
||
|
Ug to K |
||
|
K to Eth |
||
|
Ug to Eth |
||
|
not enough information |
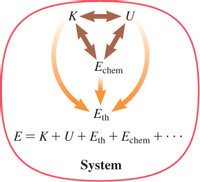
Transcribed Image Text:### Energy Transfer and Transformation in a System
This diagram illustrates the energy transfer and transformation within a system.
#### Components of the System:
- **K**: Represents Kinetic Energy (the energy of motion).
- **U**: Denotes Potential Energy (the stored energy in the system due to its position or arrangement).
- **E<sub>chem</sub>**: Symbolizes Chemical Energy (the energy stored in chemical bonds).
#### Energy Transformations:
- **Arrows between K and U**: Indicate the interchange between kinetic and potential energy, suggesting energy can shift back and forth between these forms.
- **Arrows from K and U to E<sub>chem</sub>**: Show how kinetic and potential energy can convert into chemical energy.
- **E<sub>th</sub>**: Represents Thermal Energy (heat energy).
- **Arrows from E<sub>chem</sub> to E<sub>th</sub>**: Illustrate the conversion of chemical energy into thermal energy.
#### Energy Equation:
- **E = K + U + E<sub>th</sub> + E<sub>chem</sub> + ...**: Represents the total energy of the system, which is the sum of kinetic, potential, thermal, chemical, and possibly other forms of energy.
This diagram emphasizes the dynamic nature of energy within a system and how it can continually transform from one type to another while the total energy remains constant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- An ideal gaseous reaction occurs at a constant pressure of 35.0 atm and releases 59.8 kJ of heat. Before the reaction, the volume of the system was 8.20 L. After the reaction, the volume of the system was 2.28 L. Calculate the total change in internal energy for the system. Enter your answer numerically in units of kJ.arrow_forwardA 58-kg cross-country skier glides over snow as in the figure below. The coefficient of friction between skis and snow is 0.24. Assume all the snow beneath her skis is at 0°C and that all the internal energy generated by friction is added to snow, which sticks to her skis until it melts. How far would she have to ski to melt 1.5 kg of snow? Your incorrect answer may have resulted from roundoff error. Make sure you keep extra significant figures in intermediate steps of your calculation. m Need Help? Read It Master itarrow_forward4) I need your help for question attached. note: required data is also attached.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON