
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A + B -> C
what is m and n?
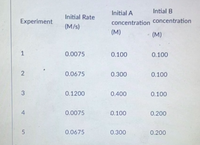
Transcribed Image Text:Initial A
Intial B
Initial Rate
Experiment
concentration concentration
(M/s)
(M)
(M)
1
0.0075
0.100
0.100
0.0675
0.300
0.100
0.1200
0.400
0.100
0.0075
0.100
0.200
0.0675
0.300
0.200
2.
3.
4)
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hon 4 x + www-awa.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IQgHIvdL2donGg9plnQUm9kiGJhghYuAiZLqdtEEgiJQTK5Ij49wHEXm8hvCYgFhBQ-k3zPGg2eq... ☆ School planner -... KH Chapter Content -... KH Chapter Content -... Q Chemistry lab Flas... Untitled documen... C ell Biology... ||| dis ne 0 O ELECTROCHEMISTRY Writing and balancing complex half-reactions in basic solution 'Low FODMAP ric... M Gmail Explanation Check 280 V Write a balanced half-reaction for the reduction of solid manganese dioxide (MnO₂) to manganese ion (Mn²+) in basic aqueous solution. Be sure to add physical state symbols where appropriate. B 000 APR 14 48 tv N 9 ローロ MacBook Air e X PN Periodic X Ś SN Periodic X AVALEPPEY (312) Yc X 1/5 A * = 334 Sabrina V 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 18 Ar 8. ) ?arrow_forward32arrow_forward(0) Mechanism NaCN скоконarrow_forward
- I need step by step process solving [4.184x156(Tf-295)] + [4.184x85.2(Tf-368)] = 0. because my work is wrong. Thank you!!arrow_forwardb Answe X e Oefeni x Oefeni x Oefeni x Oefeni x A Oefeni X 2021 x Oefeni x Micros X com/forms/d/e/1FAlpQLSfSGcE8gf218c6JoHMWME1A8Nydf8M4g1yG93D-2LPpoMj g/formResponse Figure 12 NaNH2 CH3–I H20 CH3-C=CH Hg2, H* Question 13: Consider the synthesis scheme in Figure 12. Give the preferred IUPAC name of C? [Use lowercase letters. Do not use spaces if it is not required in the name.] * Jou antwoord Show all 10:08 28°C Sunny 2021/11/19 Narrow_forwardFor a normal phase separation, predict the order of elution of the following compoundsa. n-hexane, n- hexanol, benzene, 1,2-hexadiol, 1,2-pentadiolb. ethyl acetate, diethyl ether, nitrobutane, 2-nitrobutane, 3-methyl-1-nitrobutane, 2-methyl-1-nitrobutanearrow_forward
- 148409766/materials/gp/2496058638 (5) Practice Dashb... Desmos | Graphing... Q Learn: English voca. gy - final assessment.docx ab 2 /2 > 157% 5. Calculate AH for the reaction N2H4 (1) + O2 (g) - N2 (g) + 2 H20 (I) given the following data: 2 NH3(g) + 3 N;O(g) ----> 4 N2(g) + 3 H2O(1) AH° = -1010. kJ N2O(g) + 3 H2(g) ----> N2H¾(1) + H2O(I) AH° = -317 kJ 2 NH3(g) + 1/2 O2(g) ----> N2H4(1) + H¿O(1) AH° = -143 kJ H2(g) + 1/2 O2(g) ----> H2O(1) AH° = -286 k) ** Be sure to show all of your manipulated equations. rr Sign outarrow_forwardX aw 8 Bb 67847985 4 /learn-us-east-1-prod-fleet01-xythos.content.blackboardcdn.com/blackboard.learn.xythos.prod/5a30bcf95ea52/67847985?X-Blackboard-S3-Bucket-blackboard.learn.xythos... Learn P Mastering Microbio... PE-TEXT Introductio... Gmail YouTube Maps C Get Homework Hel... Applications | Rapid..... Read aloud Ask Bing Al 3 of 4 D % 5 X Q Search ChatGPT 10 6 8. Perform a conformational analysis of the following molecule. Pay attention to the relative energies of various conformations, but do not concern yourself with the actual energy values in kJ per mole. CI Br & + 7 * x + 4+ 8 lyi K44 9 fro Mail-Mado K Ndjo... O Q Google ☆ CEL CD ☆ delete backspace @ Q homarrow_forward-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQg34RWYTxj_1SoekWMaP1As1fEhnC179H4SL2ZsgO0wePS_GkvJitHEX1MKthb4VX9coDdX5z6C9y= O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 4+ Pb 2+ Fe 2+ Zn anion 103 so² Cro Some ionic compounds empirical formula 0 0 name of compound 0 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning