
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Which C=O functional group is present in the following molecule?
(The molecule is in the picture)
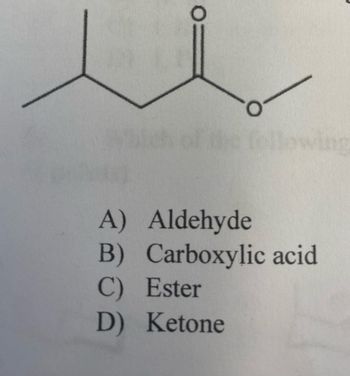
Transcribed Image Text:The image shows a structural chemical formula for an organic compound. It features a carbon chain with an ester functional group.
The structure includes:
- Three carbon atoms connected by single bonds, with the middle carbon double-bonded to an oxygen atom.
- The middle carbon is also bonded to an oxygen atom, which further connects to another carbon atom, forming an ester linkage.
Below the structure, there is a multiple-choice question asking to identify the type of functional group present in the compound:
A) Aldehyde
B) Carboxylic acid
C) Ester
D) Ketone
The correct answer is C) Ester.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. CH;CL 6. CH:Cl2 7. CH:OH (indicate the slectron pair gecmsty and molecular shaps arond both the carbon ztom znd oxygsa ztom.) 8. CO.arrow_forwardWhich would be correct please? do I separate the OH?arrow_forwardTake pictures of each step of the procedure while you are performing the experiment. Submit the complete table below and the pictures of your "toothpick-marshmallow" models indicating polarity (or not) of each molecule. Total Draw Lewis Structure How many electron How many of What is the SHAPE of humber of (follow steps): the electron the electron regions? valence groups are groups are Molecular electrons there around lone pairs? formula: (TVE): the central What is the SHAPE of atom? the molecule? 1. H2S 2. HCF3 1. (carbon is the central atom; all of the other 2. atoms are attached to C) OBR2arrow_forward
- 19. SF, 20. BrF: 21. SiO; 22. NH.CIO: (indicate the alectron pair gecmatny and molecular shapa around sacha ion.)arrow_forwardGive only typing answer with explanation and conclusion Consider the compounds below. First, identify if the compound is covalent or ionic. If covalent, identify the intermolecular forces that compound will have. Then, rank them based on their melting point – with 1 being highest, 5 being lowest. NaBr LiF H2S C2H6 H2Oarrow_forwardQuestion 3. Select the correct molecular geometry for each of the following central atoms: NH3= [Select] PCI4+ = NOCI= [Select] HNO2 (central atom = N) = [Select] HNO2 (central atom = O) = [ Select] SO3 [ Select] SO₂ = CO₂ = [Select] = [ Select] [ Select] O (> COF2= [Select] () CH3OH (central atom = C) = [ Select] ✪ CH3OH (central atom = O) = [ Select] O ◊ û NH₂CH₂CO₂H (central atom = N) = [Select] NH₂CH₂CO₂H (central atom = CH2) = [Select] NH₂CH₂CO₂H (central atom = CO2) = [Select] ✪ ◊ ✪ ✪ ✪arrow_forward
- Draw the condensed structure of an isomer of this molecule: CH3-CH2 -CH2 CH3 Click anywhere to draw the first atom of your structure.arrow_forwardIn one of the two boxes below, draw a wedge and dashed wedge structure (picture) of CH3Cl that best illustrates the geometry about the central atom. In the other box, draw another picture of the model from a different angle (viewpoint).arrow_forwardAnswer the following question.arrow_forward
- For each of the following molecules in the list: 1) List the total number of valence electrons and then draw the Lewis dot structure. 2) Use the kits to make a model of each molecule or ion. (A specific ball may not exist for the type of atom that you are looking for. If this happens, use a ball for which the number of holes matches the number of electron areas needed.) 3) Name the electron pair geometry. 4) Name the molecular shape. 5) Draw a 3-D sketch of the molecular shape and list the bond angles. 6) If the molecule contains covalent bonds, indicate if it is polar or nonpolar. 7) When necessary, draw a polar arrow for each bond. 8) If the molecule is polar, draw an arrow next to the 3-D sketch indicating the direction of the dipole moment. Molecular Lewis Dot AXE Type Electron on Central Pair Atom Molecular Ionic, polar, or nonpolar 3-D Sketch Formula Structure Shape with bond Geometry angles and dipole arrowarrow_forwardWhat does "penta-" mean? What does "di-" mean? The only exception for prefixes is when there is only one atom of the element. Example: CO is called Carbon Monoxide NOT MonoCarbon Monoxide Choose: first, secondarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY