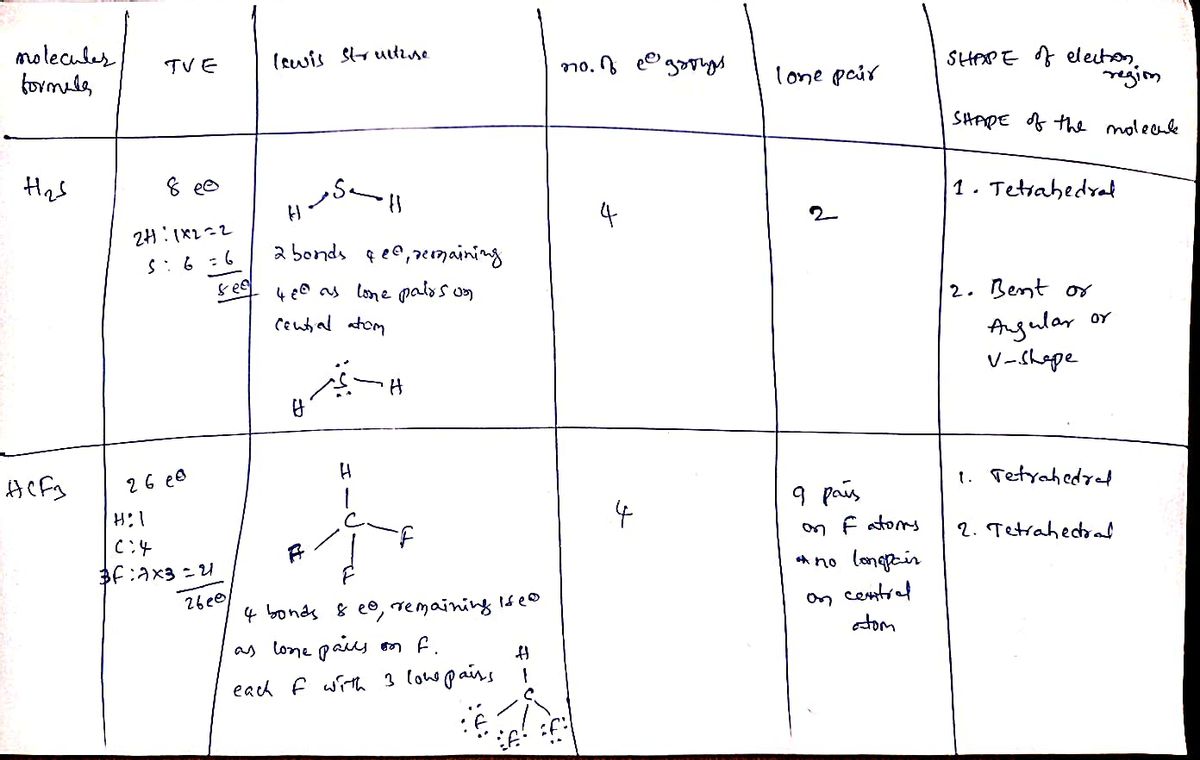
Take pictures of each step of the procedure while you are performing the experiment. Submit the complete table below and the pictures of your "toothpick-marshmallow" models indicating polarity (or not) of each molecule. Total Draw Lewis Structure How many electron How many of What is the SHAPE of the electron number of (follow steps): the electron regions? valence groups are groups are Molecular electrons there around lone pairs? formula: (TVE): the central What is the SHAPE of atom? the molecule? 1. H2S 2. HCF3 1. (carbon is the central atom; all of the other 2. atoms are attached to C) OBR2
Take pictures of each step of the procedure while you are performing the experiment. Submit the complete table below and the pictures of your "toothpick-marshmallow" models indicating polarity (or not) of each molecule. Total Draw Lewis Structure How many electron How many of What is the SHAPE of the electron number of (follow steps): the electron regions? valence groups are groups are Molecular electrons there around lone pairs? formula: (TVE): the central What is the SHAPE of atom? the molecule? 1. H2S 2. HCF3 1. (carbon is the central atom; all of the other 2. atoms are attached to C) OBR2
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 37CR
Related questions
Question

Transcribed Image Text:Take pictures of each step of the procedure while you are performing the experiment. Submit the complete table below and
the pictures of your "toothpick-marshmallow" models indicating polarity (or not) of each molecule.
Total
Draw Lewis Structure
How many
electron
How many of What is the SHAPE of
humber of (follow steps):
the electron
the electron regions?
valence
groups are
groups are
Molecular
electrons
there around lone pairs?
formula:
(TVE):
the central
What is the SHAPE of
atom?
the molecule?
1.
H2S
2.
HCF3
1.
(carbon is the
central atom;
all of the other
2.
atoms are
attached to C)
OBR2
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
