
Grignard reagents react with a variety of different compounds. What are the expected products from the following reactions?
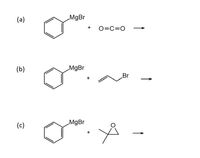
In the first reaction, Phenyl magnesium bromide is reacting with carbon dioxide. In carbon dioxide, a partial positive charge is developed on carbon while a partial negative charge will be developed on oxygen due to electronegativity difference. Electronegativity of oxygen is more than carbon so it will attract shared pair of electrons towards itself and therefore partial negative charge develops on it.
In the case of Phenyl magnesium bromide, magnesium is electropositive in nature so its electronegativity is less than carbon, therefore a partial negative charge will develop on carbon which further going to attack partial positive carbon of carbon dioxide.
Step by stepSolved in 3 steps with 1 images

- Grignard reagents react with a variety of different compounds. What are the expected products from the following reaction?arrow_forwardRank the following compounds in order of fastest reaction when treated with H2SO4 (1–5, where 1 = fastest), and explain your answer.arrow_forwardComplete the reaction schemes below providing the reagents required to achieve thetransformation. More than one step may be necessary for each scheme.arrow_forward
- Draw the expected major product for the following reaction.arrow_forwardPropose a synthesis for ONE of the following compounds in good yield from the given starting material and any other necessary reagents. The cyclopentyl group in the starting material needs to be the cyclopentyl group in the product. Hint: You will need to go through carbonyl-containing intermediates. NH₂ он orthm org NH₂arrow_forwardWhat is the final product of the multi-step synthesis sequence of reactions shown? NANH, NH; LINH; Product A Benzyl bromide (CH),Culi excess Product C Final Product Product B в Compound C Compound A Compound B A 50:50 mixture of Compounds A and B None of the above are the correct Final Productarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





