
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:A 9.00 L tank at 9.4 °C is filled with 10.7 g of dinitrogen monoxide gas and 13.1 g of carbon dioxide gas. You can assume both gases behave as ideal gases
under these conditions.
Calculate the mole fraction of each gas. Be sure each of your answer entries has the correct number of significant digits.
gas
dinitrogen monoxide
carbon dioxide
mole fraction
0
X
Expert Solution
arrow_forward
Step 1
The mole fraction of a component in the mixture is defined by the ratio of moles of that substance and the total moles of all components in the mixture.
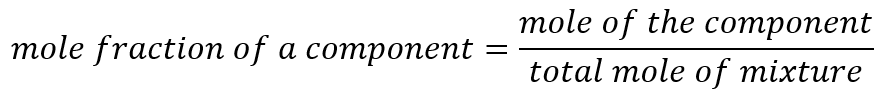
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 7.00 L tank at -10.6 °C is filled with 12.1 g of sulfur hexafluoride gas and 10.0 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas mole fraction sulfur hexafluoride dinitrogen difluoridearrow_forwardSome N, gas is mixed with some O, gas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured, and found to be 0,55 atm. key carbon hydrogen nitrogen sulfur oxygen chlorine Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to 2 significant digits. You may assume each gas behaves as an ideal gas. gas mole fraction partial pressure atm N2 || atmarrow_forwardA 7.00 L tank at 29.4 °C is filled with 2.63 g of sulfur hexafluoride gas and 12.9 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas sulfur hexafluoride boron trifluoride mole fraction 0 0 X 00- Ararrow_forward
- A 8.00 L tank at 3.9 °C is filled with 17.0 g of sulfur tetrafluoride gas and 7.06 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas mole fraction ? sulfur tetrafluoride boron trifluoridearrow_forwardPlease see imagearrow_forwardA 10.0 L tank at 13.5 °C is filled with 15.4 g of dinitrogen difluoride gas and 4.72 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: dinitrogen difluoride partial pressure: atm mole fraction: sulfur tetrafluoride partial pressure: || atm Total pressure in tank: atmarrow_forward
- Some N, gas is mixed with some O, gas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured, and found to be 1100. torr, key carbon hydrogen nitrogen sulfur охудen chlorine Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to 4 significant digits. You may assume each gas behaves as an ideal gas. gas mole fraction partial pressure N2 torr ? O2 torrarrow_forwardA 8.00 L tank at 21.1 °C is filled with 11.6 g of chlorine pentafluoride gas and 12.6 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: x10 chlorine pentafluoride partial pressure: ? atm mole fraction: sulfur tetrafluoride partial pressure: atm Total pressure in tank: atmarrow_forwardSome N, gas is mixed with some 0, gas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured, and found to be 0,120 kPa. key carbon hydrogen nitrogen sulfur охудеn chlorine Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to 3 significant digits. You may assume each gas behaves as an ideal gas. gas mole fraction partial pressure N2 | kPa O2 kPaarrow_forward
- A 7.00L tank at 0.68°C is filled with 7.59g of boron trifluoride gas and 17.5g of chlorine pentafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. boron trifluoride mole fraction: partial pressure: atm chlorine pentafluoride mole fraction: partial pressure: atmarrow_forwardA 10.00 L tank at -7.6 °C is filled with 5.09 g of chlorine pentafluoride gas and 16.3 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas chlorine pentafluoride dinitrogen difluoride mole fraction 0 10 X Sarrow_forwardCalculate the mole fraction and partial pressure of each gas as well as the total pressure in the tank using the information below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY