
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
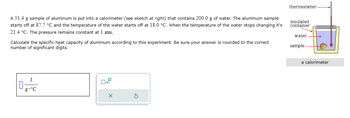
Transcribed Image Text:A 51.4 g sample of aluminum is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The aluminum sample
starts off at 87.7 °C and the temperature of the water starts off at 18.0 °C. When the temperature of the water stops changing it's
21.4 °C. The pressure remains constant at 1 atm.
Calculate the specific heat capacity of aluminum according to this experiment. Be sure your answer is rounded to the correct
number of significant digits.
0-
X
Ś
thermometer.
insulated
container
water
sample
a calorimeter
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- thermometer. A 52.7 g sample of iron is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The iron sample starts off at insulated container 94.0 °C and the temperature of the water starts off at 19.0 °C. When the temperature of the water stops changing it's 20.8 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of iron according to this experiment. Be sure your answer is rounded to 2 significant digits. sample - a calorimeter J g.°Carrow_forwardA 52.0 g sample of polystyrene is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The polystyrene sample starts off at 86.4 °C and the temperature of the water starts off at 21.0 °C. When the temperature of the water stops changing it's 26.0 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of polystyrene according to this experiment. Be sure your answer is rounded to 2 significant digits. đặc J g. °C 0 x10 X thermometer insulated. container water sample a calorimeterarrow_forwardthermometer A 53.4 g sample of iron is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The iron sample starts off at insulated container 96.3 °C and the temperature of the water starts off at 19.0 °C. When the temperature of the water stops changing it's 21.0 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of iron according to this experiment. Be sure your answer is rounded to 2 significant digits. sample a calorimeter J x10 g.°Carrow_forward
- Give typed solutionarrow_forwardA 50.5 g sample of quartz is put into a calorimeter (see sketch at right) that contains 150.0 g of water. The quartz sample starts off at 98.8 °C and the temperature of the water starts off at 15.0 °C. When the temperature of the water stops changing it's 19.5 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of quartz according to this experiment. Be sure your answer is rounded to 2 significant digits. J g. °C x10 X S thermometer. insulated container water sample a calorimeter ? olaarrow_forwardA 60.0 g sample of iron is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The iron sample starts off at 98.3 °C and the temperature of the water starts off at 22.0 °C. When the temperature of the water stops changing it's 24.1 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of iron according to this experiment. Be sure your answer is rounded to the correct number of significant digits. 0 J g. °C x10 X thermometer insulated container water sample. a calorimeterarrow_forward
- A 55.0 g sample of brass is put into a calorimeter (see sketch at right) that contains 100.0 g of water. The brass sample starts off at 86.7 °C and the temperature of the water starts off at 23.0 °C. When the temperature of the water stops changing it's 26.5 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of brass according to this experiment. Be sure your answer is rounded to the correct number of significant digits. J 0- g-°C 0 x10 X thermometer. insulated container water sample a calorimeterarrow_forwardA 30.0 g sample of chromium is heated to 100.0 °C in a boiling water bath. The sample is added to a calorimeter charged with 45.0 mL of water at 34.1 °C. The final temperature of the calorimeter contents is 38.5 °C. Calculate the specific heat of chromium.arrow_forwardConsider two 50.0 mL volumes of water, one at 20.0 °C and the other at 100.0 °C. The temperature of the combined solutions when mixed in a calorimeter is measured to be 52.4 °C. What are the temperature changes for each of the volumes of water?arrow_forward
- thermometer A 59.2 g sample of iron is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The iron sample starts off at 95.8 °C and the temperature of the water starts off at 24.0 °C. When the temperature of the water stops changing it's 27.1 °C. insulated container The pressure remains constant at 1 atm. water Calculate the specific heat capacity of iron according to this experiment. Be sure your answer is rounded to 2 significant digits. sample a calorimeter g.°C Explanation Check O2021 McGraw-Hill Education. All Rights Reserved Terms of Usse Privacy Accessibi MacBook Aiarrow_forwardA 56.5 g sample of iron is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The iron sample starts off at 86.7 °C and the temperature of the water starts off at 21.0 °C. When the temperature of the water stops changing it's 22.8 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of iron according to this experiment. Be sure your answer is rounded to 2 significant digits. J 0₂-C x10 x thermometer insulated container water sample a calorimeterarrow_forwardA 54.5 g sample of brass is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The brass sample starts off at 95.8 °C and the temperature of the water starts off at 15.0 °C. When the temperature of the water stops changing it's 16.4 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of brass according to this experiment. Be sure your answer is rounded to 2 significant digits. П J g.°C x10 X S thermometer insulated container water sample a calorimeter U!!! 0 Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY