
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
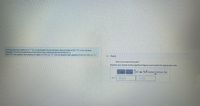
Transcribed Image Text:A 33.0 g iron rod, initially at 21.7°C, is submerged into an unknown mass of water at 63.3 °C, in an insulated
container. The final temperature of the mixture upon reaching thermal equilibrium is
58.0°C. The specific heat capacity of water is 4.184 Jg cand the specific heat capacity of iron is 0.449 Jg C
- Part A
What is the mass of the water?
Express your answer to two significant figures and include the appropriate units.
Templates Symbols undo redo Tese keyboard shortcuts Help
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 32.5-g iron rod, initially at 22.7 °C, is submerged into an unknown mass of water at 63.2 °C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 59.5 °C. What is the mass of the water?arrow_forwardA 20.0 g block of iron (molar heat capacity 25.1 J/mol・°C) at 36.6 °C is placed into 200.0 g of water initially at 25.0 °C. What is the change in temperature (in °C) of the iron block? (The molar heat capacity of water is 75.38 J/mol・°C).arrow_forwardA 138.9 g sample of an unknown metal at a temperature of 99.1 °C is added to 44.72 g of water at a temperature of 19.1 °C. The specific heat of water is 4.184 Jg-1 °C- 1. The final temperature of the system is 52.2°C. Calculate the specific heat of the unknown metal.arrow_forward
- A piece of titanium metal with a mass of 21.6 g is heated in boiling water to 99.5 °C and then dropped into a coffee-cup calorimeter containing 75.0 g of water at 21.8 °C. When thermal equilibrium is reached, the final temperature is 24.5 °C. Calculate the specific heat capacity of titanium. (The specific heat capacity of liquid water is 4.184 J/g. K.) Specific heat capacity= J/g. Karrow_forwardA 79.1 g sample of an unknown solid is heated to 62.6ºC and placed into a calorimeter containing 92.1 g of water at 22ºC. If the final temperature of the solid sample and the water is 32.5ºC, what is the specific heat of the solid? Please provide your final answer rounded to two decimal places.arrow_forwardA 53.0-g metal weight, heated to 89.50°C, is placed into 133 g of water at 20.65°C contained in a perfectly insulating thermos flask. After some time, the temperature inside the thermos flask stabilizes at 23.10°C. The specific heat capacity of water is approximately 4.18 J/K/g in the temperature range 16°C - 61°C.Calculate the specific heat capacity of the metal.arrow_forward
- A 149.2-g sample of a metal at 74.4°C is added to 149.2 g H20 at 15.5°C. The temperature of the water rises to 18.6°C. Calculate the specific heat capacity of the metal, assuming that all the heat lost by the metal is gained by the water. The specific heat capacity of water is 4.18 J/°C g. Specific heat capacity = | J/°C•&arrow_forwardWhen a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. Thermometer Cardboard or In the laboratory a general chemistry student finds that when 3.38 g of CuCl,(s) are Styrofoam lid dissolved in 114.10 g of water, the temperature of the solution increases from 25.37 to 27.99 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.60 J/°C. Nested Styrofoam cups Based on the student's observation, calculate the enthalpy of dissolution of CuCl2(s) in kJ/mol. - Reaction occurs in Assume the specific heat of the solution is equal to the specific heat of water. solution. AHdissolution kJ/mol kc Cengage Lnarrow_forwardPlease don't provide handwritten solution ...arrow_forward
- A 44.0 g sample of unknown metal at 99.0°C was placed in a constant-pressure calorimeter containing 80.0 g of water at 24.0°C, the final temperature of the system was found to be 28.4°C. Calculate the specific heat of the metal.arrow_forwardPart A A silver block, initially at 55.8 °C, is submerged into 100.0 g of water at 24.1°C in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 26.3 °C. The specific heat capacities for water and silver are = 4.18 J/(g ·°C) and 0.235 J/(g·°C). What is the mass of the silver block? Express your answer to two significant figures and include the appropriate units. Cs, water • View Available Hint(s) Cs, silver = HA ? Value Units m =arrow_forwardWhen a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. Thermometer Cardboard or In the laboratory a general chemistry student finds that when 0.46 g of MgCl,(s) are Styrofoam lid dissolved in 102.20 g of water, the temperature of the solution increases from 24.17 to 26.04 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.67 J/°C. Nested Styrofoam cups Based on the student's observation, calculate the enthalpy of dissolution of MgCl2(s) in kJ/mol. Reaction occurs in Assume the specific heat of the solution is equal to the specific heat of water. solution. AHdissolution kJ/mol chookc Cngage Lngarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY