
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Need answer ASAP!
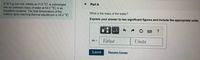
Transcribed Image Text:A 32.4 g iron rod, initially at 21.9 °C, is submerged
into an unknown mass of water at 64.0 °C, in an
insulated container. The final temperature of the
mixture upon reaching thermal equilibrium is 59.4 °C.
Part A
What is the mass of the water?
Express your answer to two significant figures and include the appropriate units.
HA
Value
Units
m =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The students conducted the assay for LDH activity using the two serum samples. Each cuvette (path length 1 cm) contained 3ml of a suitable assay buffer (including pyruvate as substrate). 20 microlitres of either serum was added to the cuvette and the absorbance values immediately recorded at the optimum wavelength for a period of 5 minutes (absorbance readings taken every 30 seconds). Protein concentration of serum sample (mg/ml) Change in absorbance at optimum wavelength per minute Control serum (C) 8 -0.04 Diseased serum (D) 7.8 -0.6 1c. Using the molar absorption coefficient of NADH as 6220 M-1 cm-1, and by application of the Beer-Lambert law, estimate the enzyme activity in the two samples (C and D). Express activity as moles per second. 1d. Estimate the specific activity of the two samples (moles per second per microgram).arrow_forwardS $24 CHEM 107-02 Exam 1: Ju x → A ALEKS - Julianna Graham - L x + www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QlyULO-QdKyW_57MDR1Qpi-ijkNcyRp81fjPxufK3ckTA8G12 Td... O Acids and Bases Calculating the pH of a strong acid solution 0/3 Julianna A chemist dissolves 855. mg of pure perchloric acid in enough water to make up 250. mL of solution. Calculate the pH of the solution. Round your answer to 3 significant decimal places. 0 Explanation Check G MacBook Air olo 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardAnalytes than can be trapped inside Sephadex G-25 (1,000-5000) Cytochrome C (MW=12384) Aprotinin (MW=6512) Vitamin B12 (MW=1355) Gly-gly-gly (MW=189)arrow_forward
- Need full case study with results discussion Introduction etc. Dont use guideline answer ok need full accurate case study. Case History A 32 year old male was found unconscious in his flat following a night out with friends. Attempts to resuscitate the male were unsuccessful. The postmortem indicated sudden cardiac death through cardiac dysrhythmia (arrythmia). There was no medical history of previous heart disease. Two opened packets of Jumping Beans were found on a table close to the body. A specimen of cardiac blood was taken and submitted to the toxicology laboratory for analysis along with the two Jumping Beans packets. . Guidance for Preparing the Case Report The coursework assessment for the module is to prepare a case report in the form of a case report scientific paper published in the Journal of Analytical Toxicology. This guidance information has been taken from the Instructions for Authors for the Journal of Analytical Toxicology however please use the…arrow_forwardWhat kind of electrophysiological experiment is illustrated in the recordings depicted below?arrow_forward6829Cu->0-1beta+?arrow_forward
- Benzocaine has been tested as an anaesthetic for inhibition of m1 muscarinic acetylcholine signalling. Using the data below, plot a graph and determine the IC50. Concentration (M) 1 x 10-6 10 x 10-6 100 x 10-6 1000 x 10-6 10000 x 10-6 100000 x 10-6 % of control response 97 93 78 56 42 36arrow_forwardHow would you solve 24?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY