
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![### Problem:
A 3.000 L balloon is heated from 3000.0 K to 4000.0 K at a pressure of 1.900 atm. What is the new volume of the balloon?
### Step-by-Step Solution:
#### First:
Identify the type of problem:
**This is a:**
- [ ] a. missing variable
- [x] b. change in conditions
- [ ] c. 22.414 L/1 mol
- [ ] d. density = molar mass/22.414 L
- [ ] e. partial P
#### Second:
Determine what needs solving:
**We need to solve for:**
- [x] k. V₂ (not V)
**Using the equation:**
- v. \[ \frac{P_1V_1}{n_1T_1} = \frac{P_2V_2}{n_2T_2} \]
### Information and Formulas:
- **Symbols and values:**
- f. P
- g. P₁
- h. P₂
- i. V
- j. V₁ (not V)
- l. n
- m. n₁ (not n)
- n. n₂ (not n)
- o. T
- p. T₁ (not T)
- q. T₂ (not T)
- r. R = 8.205 x 10⁻² L atm/K mol
- s. 1 atm = 760 torr
- **Total Pressure:**
- t. \( P_{\text{total}} = P_1 + P_2 + \ldots \)
- **Ideal Gas Law:**
- u. PV = nRT
### Finally:
Select the correct answer for the new volume:
- v. \[4.000 L\]
- w. \[2.250 L\]
- x. \[0.7500 L\]
- y. **1.333 L**](https://content.bartleby.com/qna-images/question/3af619a8-05d7-4a08-b6ab-836abf944ec6/9991736e-0b11-42d8-8dda-6e6232b61385/66j38mt_thumbnail.png)
Transcribed Image Text:### Problem:
A 3.000 L balloon is heated from 3000.0 K to 4000.0 K at a pressure of 1.900 atm. What is the new volume of the balloon?
### Step-by-Step Solution:
#### First:
Identify the type of problem:
**This is a:**
- [ ] a. missing variable
- [x] b. change in conditions
- [ ] c. 22.414 L/1 mol
- [ ] d. density = molar mass/22.414 L
- [ ] e. partial P
#### Second:
Determine what needs solving:
**We need to solve for:**
- [x] k. V₂ (not V)
**Using the equation:**
- v. \[ \frac{P_1V_1}{n_1T_1} = \frac{P_2V_2}{n_2T_2} \]
### Information and Formulas:
- **Symbols and values:**
- f. P
- g. P₁
- h. P₂
- i. V
- j. V₁ (not V)
- l. n
- m. n₁ (not n)
- n. n₂ (not n)
- o. T
- p. T₁ (not T)
- q. T₂ (not T)
- r. R = 8.205 x 10⁻² L atm/K mol
- s. 1 atm = 760 torr
- **Total Pressure:**
- t. \( P_{\text{total}} = P_1 + P_2 + \ldots \)
- **Ideal Gas Law:**
- u. PV = nRT
### Finally:
Select the correct answer for the new volume:
- v. \[4.000 L\]
- w. \[2.250 L\]
- x. \[0.7500 L\]
- y. **1.333 L**
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
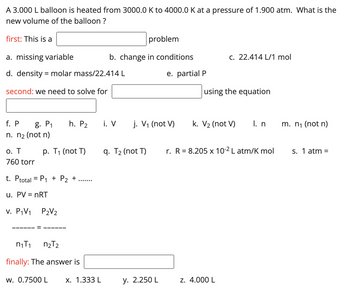
Transcribed Image Text:A 3.000 L balloon is heated from 3000.0 K to 4000.0 K at a pressure of 1.900 atm. What is the
new volume of the balloon ?
first: This is a
a. missing variable
d. density = molar mass/22.414 L
second: we need to solve for
o. T
760 torr
f. P g. P₁ h. P₂ i. V j. V₁ (not V)
n. n₂ (not n)
p. T₁ (not T)
t. Ptotal = P₁ + P₂ +
u. PV = nRT
v. P₁V₁
P2V₂
n₁T₁
n₂T2
finally: The answer is
w. 0.7500 L
problem
b. change in conditions
x. 1.333 L
q. T₂ (not T)
e. partial P
y. 2.250 L
c. 22.414 L/1 mol
using the equation
k. V₂ (not V)
z. 4.000 L
I. n
r. R = 8.205 x 10-² L atm/K mol
m. n₁ (not n)
s. 1 atm =
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:A 3.000 L balloon is heated from 3000.0 K to 4000.0 K at a pressure of 1.900 atm. What is the
new volume of the balloon ?
first: This is a
a. missing variable
d. density = molar mass/22.414 L
second: we need to solve for
o. T
760 torr
f. P g. P₁ h. P₂ i. V j. V₁ (not V)
n. n₂ (not n)
p. T₁ (not T)
t. Ptotal = P₁ + P₂ +
u. PV = nRT
v. P₁V₁
P2V₂
n₁T₁
n₂T2
finally: The answer is
w. 0.7500 L
problem
b. change in conditions
x. 1.333 L
q. T₂ (not T)
e. partial P
y. 2.250 L
c. 22.414 L/1 mol
using the equation
k. V₂ (not V)
z. 4.000 L
I. n
r. R = 8.205 x 10-² L atm/K mol
m. n₁ (not n)
s. 1 atm =
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An experiment starts with an ideal-gas sample in a sealed container with a movable piston at initial pressure of Po, initial temperature 300 K and initial volume 8 L. In the experiment, the temperature is increased to 450 K, while the piston is moved until the volume decreases to 4 L. What will the final pressure be equal to? ○ / Po 23 & Po 2Po 3Po 32 6Poarrow_forwardpls help asap on all asked questions.arrow_forwardConsider equimolar samples of different ideal gases at the same volume and temperature. Gas A has a higher molar mass than gas B. Compare the pressures. A>B A=B A<B Compare the rms speeds. A>B A=B A<B Compare the average kinetic energies. A>B A=B A<B Consider equimolar samples of the same ideal gas at the same volume, but different temperatures. Sample C is at a higher temperature than sample D. Compare the pressures. C>D C=D C<D Compare the rms speeds. C>D C=D C<D Compare the average kinetic energies. C>D C=D C<D Consider equimolar samples of the same ideal gas at the same temperature, but different volumes. Sample E has a larger volume than sample F. Compare the pressures. E>F E=F E<F Compare the rms speeds. E>F E=F E<F Compare the average kinetic energies. E>F E=F E<Farrow_forward
- #10 for the hw please show workarrow_forwardA 0.1 mL gas bubble forms deep in the bottom of a lake, where the pressure on the bubble is 4.4 atm. What will be the volume of the bubble at the surface of the lake, where the atmospheric pressure is 728 mm Hg? (constant T and n)arrow_forwardA 1.59 mol sample of Xe gas is confined in a 38.9 liter container at 25.3 degrees celcius. If the temperature of the gas sample is raised to 45.5 degrees celcius holding the volume constant, the average molecular speed will: a. Remain the seme b. Increase c. Not enough information d. Decrease choose correct answer.arrow_forward
- 11. At an altitude of 40 km above the earth's surface, the atmospheric pressure is 5.00 torr, and the surrounding temperature is -20°C. If a weather balloon is filled with 1.000 mol of He at 760 torr and 22°C, what is its a. initial volume before ascent? b. final volume when it reaches 40 km in altitude? (Assume the pressure of the gas equals the surrounding pressure.)arrow_forwardPlease help me set up and solve #7 as done in top problemarrow_forwardF8.og of fluorine aas and 50g of neon gas are injecied in to a 1.8 L vessel at a temperature of 59 °C, what will the partial pressure of each gas be? What will the total pressure in the container be? Assume each gas behaves ideally.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY