
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
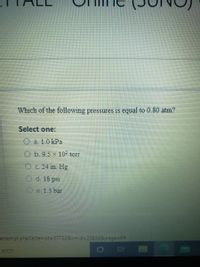
Transcribed Image Text:Which of the following pressures is equal to 0.80 atm?
Select one:
O a. 1.0 kPa
O b.9.5 x 10² torr
OC. 24 in. Hg
O d. 18 psi
O e. 1.3 bar
attempt php?attempt=37758&cmid%3D20834&page=D6%3
earch
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- How must the absolute temperature of a gas be changed to double its volume at constant pressure?a. doubledb. halvedc. quadrupledd. none of the above Would it be A. Doubled?arrow_forwardThree samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -5.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 0.99 0.70 0.73 volume (L) 20.0 25.0 30.0 ideal? O yes O no O yes O no O yes O no If not ideal, the most important reason why not: O There are attractions between the particles. The particles don't have zero volume. There are attractions between the particles. O The particles don't have zero volume. O There are attractions between the particles. O The particles don't have zero volume.arrow_forwardA10.0L gas cylinder contains the following mixture of gas. 15.00 g of N2 @ a partial pressure 0 65 atm. Xexon gas is added to the flask such that the moles of Xe and the m9les of O2 are the same. What is the new total pressure of the flask?arrow_forward
- Stales af Mal Solving applications of Boyle's Law A gas storage cylinder in an ordinary chemical laboratory measures 2.9 cm wide and 12 cm high. This is the label on it. Contents: N, gas Pressure: 7.95 atm Samanta If the cylinder is opened and the gas allowed to escape into a large empty plastic bag, what will be the final volume of nitrogen gas, including what's collected in the plastic bag and what's left over in the cylinder? Write your answer in liters. Be sure your answer has the correct number of significant digits. 0₁. 0.8 DEB 100arrow_forward7arrow_forwardThree samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -5.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 1.1 0.59 0.50 volume (L) 20.0 30.0 40.0 ideal? yes O no O yes O no O yes O no If not ideal, the most important reason why not: O There are attractions between the particles. O The particles don't have zero volume. There are attractions between the particles. O The particles don't have zero volume. O There are attractions between the particles. O The particles don't have zero volume. X 5arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY