Question
A hypothetical atom (Fig. ) has energy levels at 0.00 eV (the ground level), 1.00 eV, and 3.00 eV. (a) What are the frequencies and wavelengths of the spectral lines this atom can emit when excited? (b) What wavelengths can this atom absorb if it is in its ground level?
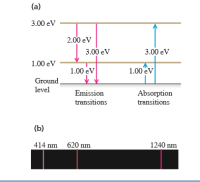
Transcribed Image Text:(a)
3.00 eV
2.00 eV
3.00 eV
3.00 eV
1.00 eV
1.00 eV
1.00 eV
Ground,
level
Emission
Absorption
transitions
transitions
(b)
414 nm 620 nm
1240 nm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- What is the energy in eV and wavelength in µm of a photon that, when absorbed by a hydrogen atom, could cause a transition from the n = 4 to the n = 6 energy level? (a) energy in eV? (b) wavelength in µm?arrow_forwardConsider photons incident on a hydrogen atom. (a) A transition from the n = 4 to the n = 7 excited-state requires the absorption of a photon of what minimum energy? eV(b) A transition from the n = 1 ground state to the n = 6 excited state requires the absorption of a photon of what minimum energy? eVarrow_forwardThe energy difference between the 1st excited state (n = 2) and the 2nd excited state (n = 3) in the hydrogen atom is 1.9 eV, what is the wavelength of the emission line resulting from the electron transitions between those two levels? Give your answer in units of nanometers (nm).arrow_forward
- Suppose you recently discovered a hydrogen like element that has only one electron orbiting around a nucleus containing a proton and a neutron. You found the ground state energy of the electron to be -16 eV. What will be the energy of this electron when it is on the excited state shown in the sketch? Note that all other possible intermediate states are shown by dashed lines. Electron is here Ground state 1.0 eV 16 eV - 1.0 eV -4.0 eV 4.0 eVarrow_forwardDetermine the distance between the electron and proton in an atom if the potential energy ?U of the electron is 16 eV (electronvolt, 1 eV =1.6×10−19=1.6×10−19 J). Give your answer in Angstrom (1 A = 10-10 m).arrow_forwardWhat is the longest - wavelength line in nanometers in the infrared series for hydrogen where m = 3?arrow_forward
- An electron within the hydrogen atom is excited from n1 to n4. The electron then "falls" back to ni in two steps (n4 -> n2, n2 -> n1). Which of the following statements is true about the light emitted during this process? One photons will be emitted. Two photons will be emitted. O Three photons will be emitted. Four photons will be emitted.arrow_forwardWhat is the energy in ev and wavelength in um of a photon that, when absorbed by a hydrogen atom, could cause a transition from the n = 5 to the n = 8 energy level? HINT (a) energy in ev ev (b) wavelength in um umarrow_forward
arrow_back_ios
arrow_forward_ios