
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
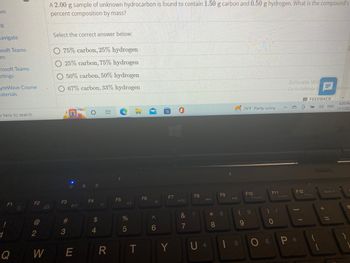
Transcribed Image Text:**Problem:**
A 2.00 g sample of unknown hydrocarbon is found to contain 1.50 g of carbon and 0.50 g of hydrogen. What is the compound’s percent composition by mass?
**Options:**
Select the correct answer below:
- 75% carbon, 25% hydrogen
- 25% carbon, 75% hydrogen
- 50% carbon, 50% hydrogen
- 67% carbon, 33% hydrogen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use percent composition and molar mass to determine molecular formula. Close Problem Question Content Area Analysis of a compound of carbon, hydrogen and oxygen showed that it is 59.94% C and 13.44% H, with O accounting for the remainder. In a separate experiment, the molar mass of the compound was found to be 60.11 g/mol. Determine the molecular formula of the compound. Enter the elementsin the order C, H, O.arrow_forwardQuestion attachedarrow_forwardUse percent composition and molar mass to determine molecular formula. Close Problem Question Content Area Analysis of a compound of chlorine, oxygen and fluorine showed that it is 34.60% Cl and 46.85% O, with F accounting for the remainder. In a separate experiment, the molar mass of the compound was found to be 102.5 g/mol. Determine the molecular formula of the compound. Enter the elementsin the order Cl, O, F.arrow_forward
- (Correct!) What is the mass of carbon dioxide that contains the same number of atoms as 10.0 g of H₂SO3? 21.5 g 24.2 g 19.8 g 15.7 g 10.7 g (Your correct answer)arrow_forwardProblem A sample of an unknown compound contains 0.21 mol of zinc, 0.14 mol of phosphorus, and 0.56 mol of oxygen. What is the empirical formula?Plan We are given the amount (mol) of each element as fractions. We use these fractional amounts directly in a preliminary formula as subscripts of the element symbols. Then, we convert the fractions to whole numbers.arrow_forwardHelp ost comr X AHRQ's Healt X Illinois StateL X Mind Tap - 55750828934189288909969212&elSBN=9781305657... ☆ Q Search this References Use the References to access important values if needed for this question. A compound is found to contain 37.23 % carbon, 7.827 % hydrogen, and 54.94 % chlorine by mass. What is the empirical formula for this compound? To answer the question, enter the elements in the order presented above. Submit Answerarrow_forward
- Please help. I have gotten the first answer right please help with the second.arrow_forwardDonarrow_forwardDetermine the molecular and empirical formulas for the substance shown in the ball-and-stick model below. Submit Answer Retry Entire Group Molecular Formula It needed for this question. Empirical Formula C = black H = gray CI = green 9 more group attempts remainingarrow_forward[References] with the empirical formula CH, was found to have a molar mass of approximately 84 g. What is the molecular formula of the compound? ements in the order: C, H.) ormula: swer Try Another Version 1 item attempt remainingarrow_forward2. Theoretically, should the experimental mass percent water values for everyone who used the same hydrate be approximately the same value? Why or why not?arrow_forwardHelp!!arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning