
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Practice chemistry question
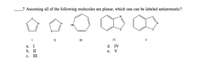
Transcribed Image Text:_7 Assuming all of the following molecules are planar, which one can be labeled antiaromatic?
HN
I
II
III
IV
а. I
d. IV
b. П
е.
V
с.
III
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many of the following molecules will rotate plane polarized light? H CH3 CH3 Ме CH3 CH2CH3 H3C. CH2CH3 CI H;C. H. ČH3 Br ci' H H3CH2C 1 2 3 4 5arrow_forwardHow many of the following molecules will rotate plane polarized light? H. CH3 CH3 CH2CH3 Me H3C, H CH3 CH2CH3 H;C, CI cı' H H3CH2C H. ČH3 Br 1 2 4 5arrow_forward88 5. Draw the following molecules in two difference configurations about the double bond. Name the molecules and be sure to include the appropriate configuration (trans or cis). If the structure cannot be drawn in two different configurations, explain why. a. Н2С-СН2 b. CH3CH=CHCH3 c. Cl(CH3)C=CHCH3arrow_forward
- 2. Assign the R or S configuration to each chiral center in the molecules below. CH2CH2B. CH3 .CO2H (H3C)3C. C=CH HO CH,F H,C=G+CH(CH,CH3)2 CH2CH,CH3 HO H C(CH3)3 ОН CH,CH,Br F"CH2Br 3 4 (numbers indicate the IUPAC numbering)arrow_forwardHow many of the following molecules will rotate plane polarized light? H. CH, CH3 Me H. CH, CH,CH3 H;C. CH2CH3 H;C. H. ČH, Br cı' H H;CH,C 2arrow_forward2. How many chiral centers does the following compound have? Br A. 2 В. 3 С.4 D. 5arrow_forward
- Which of the following molecules has a chiral (asymmetric) carbon atom? Br CH3 CH3 CH;CH2CHCH3 CH3-C ; CH3 C-CH,Br CH3CH2CH2CH2Br -Br CH3 CH3 A O a. D O b. C О с. В O d. Aarrow_forwardWhich of the following is a chiral molecule? Но- HO. O., 1 2 O A 1 O B. 2 OC.3 O D.4 3.arrow_forward4. Optical isomerism occurs when a molecule's mirror image is superimposable. b. both cis and trans isomers exist in equal concentrations. two or more structural isomers of a molecule exist. а. с. d. at least one carbon atom in a molecule is bonded to four different atoms or groups. e. both enantiomers are present in a racemic mixture.arrow_forward
- 다음 반응을 완결하시오.arrow_forward2. Identify the functional groups labelled A-I. HO, CO2H HOʻ но. HO, CO2H A HO H „CH3 H3č „CH3 H H3C° но E F Zocor (Merck) Pravachol (Bristol-Myers Squibb) Lipitor (Pfizer) FUNCTIONAL GROUPS A. В. C. D. Е. F. G. Н. I.arrow_forwardWhich OH groups are bonded to sp3 hybridized carbon atoms and which are bonded to sp2 hybridized carbons?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
