
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
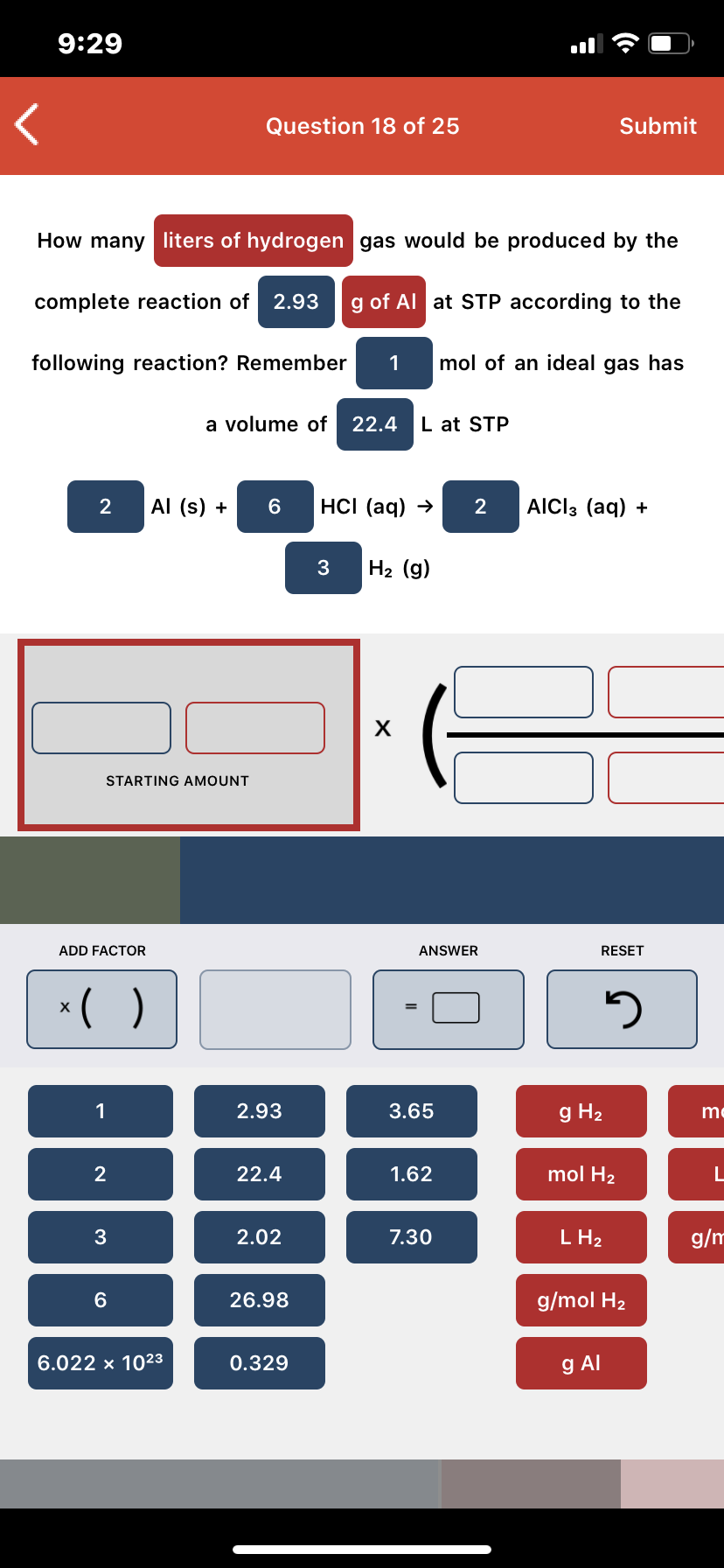
Transcribed Image Text:9:29
Question 18 of 25
Submit
How many liters of hydrogen gas would be produced by the
complete reaction of 2.93
g of Al at STP according to the
following reaction? Remember
mol of an ideal gas has
a volume of
22.4
L at STP
Al (s) +
HCI (aq) →
AICI3 (aq) +
H2 (g)
х
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
2.93
3.65
g H2
me
22.4
1.62
mol H2
2.02
7.30
L H2
g/m
26.98
g/mol H2
6.022 x 1023
0.329
g Al
2.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- PC15 (s) + H₂O(1) POC13 (1) + 2HCl(aq) When 56.39 g of phosphorus pentachloride reacts with excess water, what mass of hydrogen chloride will be produced? If needed, enter scientific notation with the "e". For example, 1.44×107 would be entered as 1.44e7. Answer: g HCI.arrow_forwardPlease help...the current balanced equation is incorrectarrow_forwardApply the significant figure rules to the final answer. The rules are attached. The final answer will not be 5.73.arrow_forward
- If a pOH meter is placed in a 0.00300 mol/L solution of nitric acid, the POH reading would be: O 3.00 O 11.5 O 0.500 O 0.300 O 2.50arrow_forwardIf a 1.101 g sample of an unknown organic molecule that contains C, H, and Cl is burned with excess oxygen gas to produce 1.977g of CO2 and 0.268 g of H2O by the generalized reaction below C[a] H[b] Cl[c] + excess O2 = xCO2+ yH2O+zCl2 Determine the mass % of Cl in the unknownarrow_forward6:18 A a0 Question 2 of 8 Submit How many moles of aluminum are required to completely react with 107 mL of 6.00 M H,SO, according to the balanced chemical reaction: 2 Al(s) + H,SO,(aq) → Al,(So,),(aq) + 3 |H2(g) TARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 98.08 6.00 428 0.0374 m 1000 0.963 107 2 1 0.001 6.022 x 1023 3 0.428 IIarrow_forward
- Calculate the number of moles of water in a solution that contains 16 g NaOH in 320 mL water. (a) 176 moles (b) 17.6 moles (c) 1.76 moles (d) 0.176 moles (d)arrow_forwardRemaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forwardQUESTION 6 How many grams of water are needed to react with 18.2 g of Mg 3N 2 in the following reaction? Mg 3N 2 + 6H20 --> 3 Mg(OH)2 + 2 NH 3arrow_forward
- A Login My AP Login - Coll. LanguageTool -Onl.. Co Biography of Albert. Crillegnettouth Pre-AP Unit 3 Learning Checkpolnt 2 1 (3 10 11 Question 9 D CH, (g) + 2 O2 (g) CO, (g) + 2 H20 (g) When CH, (g) is burned in O,(g), the reaction represented by the equation occurs. If 32 g of CH, is burned completely, how many moles of CO, are produced? Enter the number of moles to the nearest whole number. molarrow_forwardplease answer questionarrow_forwardQuestion 16 of 40 Write the balanced NET ionic equation for the reaction when aqueous BaCk and aqueous (NH4)2SO4 are mixed in solution to form aqueous NH.CI and solid BaSO4. 04 3- 2+ 3+ 4+ 1 2. 3 4. 6. 8. 9. 0. 13 (s) (1) (g) (aq) Ba CI hp 7. 1Larrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY