
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
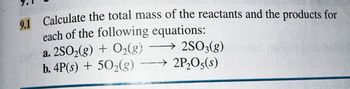
Transcribed Image Text:9.1 Calculate the total mass of the reactants and the products for
each of the following equations:
a. 2SO₂(g) + O₂(g)2SO3(g)
b. 4P(s) + 50₂(g) 2P₂O5(s)
noge
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8. Consider the following chemical reaction: 4 HBr(g) + O2(g) → 2 H2O(g) + 2 Br2(g) Identify the substance oxidized (SO), the substance reduced (SR), the oxidizing agent (OA) and the reducing agent (RA). SO: SR: OA: RA: 9. Lead (II) iodide is a bright yellow pigment made from the precipitation reaction between solutions of lead (II) nitrate and sodium iodide. a. Write the balanced equation for the precipitation reaction. b. If 19.6 g of lead (II) nitrate are mixed with 25.3 g of sodium iodide, what is the limiting reactant? Calculate and explain. С. How many grams of precipitate are formed? d. If 35.1 g of product is recovered in lab, what is the percent yield for this reaction?arrow_forwardBalance the following chemical equation (if necessary): C,H(g) + O,(g) –→ H̟O(g) + CO,(g) 04- 3. D2+ 3+ O4+ 1 4 6. 8. 9. 9. (s) (1) (g) (aq) CH0 CO, H̟O Reset • x H,O Delete 5arrow_forwardDimethyl ether, a useful organic solvent, is prepared in two steps. In the first step, carbon dioxide and hydrogen react to form methanol and water: CO₂(g) + 3 H₂(g) CH₂OH(1) + H₂O(1) In the second step, methanol reacts to form dimethyl ether and water: 2 CH₂OH(1)→ CH₂OCH3(g) + H₂O(1) Calculate the net change in enthalpy for the formation of one mole of dimethyl ether from carbon dioxide and hydrogen from these reactions. Round your answer to the nearest kJ. เม kJ AH=-131. kJ Ś ΔΗ= 8. kJarrow_forward
- 3. Determine the enthalpy change (AH₂) for the final reaction below. a. CO(g) + ½ O₂(g) → CO₂(g) AH, -67.6 kcal b. N₂(g) + O₂(g) →→→ 2NO(g) AH₂ = 43.2 kcal c. CO(g) + NO(g) →→→ CO₂(g) + ¹½ N₂(g) AH₂ = ?arrow_forward1)isothermic,exothermic, or endothermic 2)combustion,descombotion,synthesis,acid-base,double replacement,or single replacementarrow_forward5. When 5.0 moles of potassium metal reacts with an excess of H₂0, 3.5 g of H2 are produced. 2 K (s) + 2 H20 (1) 2 KOH (aq) H₂ (g) a. Calculate the number of moles of water needed to react with all of the potassium metal. (Ans: 5 mol) + b. Calculate the theoretical yield of H2, in grams, formed when all 5.0 moles of potassium metal are consumed. (Ans: 5.04 g) c. Calculate the percent yield of the reaction, given 3.5 g (actual yield) are produced. (Ans: 69.4%)arrow_forward
- Chlorine is used by textile manufacturers to bleach cloth. Excess chlorine is destroyed by its reaction with sodium thiosulfate, Na2S2O3, as follows:Na2S2O3(aq) + 4Cl2(g) + 5H2O 2NaHSO4(aq) + 8HCl(aq) Using the concepts established in the previous parts, answer the following questions about the following reaction:The octane in gasoline burns according to the following equation:2C8H18 + 25O2 → 16CO2 + 18H2O(a) How many moles of O2 are needed to react fully with 6.84 mol of octane? (b) How many moles of CO2 can form from 0.511 mol of octane? (c) How many moles of water are produced by the combustion of 8.20 mol of octane? (d) If this reaction is used to synthesize 6.00 mol of CO2, how many moles of oxygen are needed? How many moles of octane are needed? ? mol O2 ? mol C8H18 Thanks.arrow_forwardLl.8.arrow_forwardesc Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. In the first step, phosphorus and oxygen react to form diphosphorus pentoxide: P₁(1)+50₂(g) 2 P₂O(g) In the second step, diphosphorus pentoxide and water react to form phosphoric acid: P₂O(g) + 3 H₂O(1)-2 H₂PO₂(0) 4 Write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. Be sure your equation is balanced. 0 Continue 56°F Clear F1 J F2 @ 2 F3 # 3 Q Search F4 $ 4 F5 % 5 F6 6 0-0 X F7 & 00 7 Ś F8 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibi M OO DELL F9 prt sc F10 home F11 end 12 F12 0 Submit Assignment J olo 18 Ar insertarrow_forward
- Consider the following balanced reaction. How many grams of water are required to form 75.9 g of HNO3? Assume there is excess NO2 present. The molar masses are as follows: H2O = 18.02 g/mol, HNO3 = 63.02 g/mol. 3 NO2 (g) + H2O (l) ⟶2 HNO3 (aq) + NO (g) Group of answer choicesarrow_forward15. Titanium (IV) chloride is reduced by magnesium to produce titanium according to the following reaction. a. TiCla(s) + 2Mg(s) → Ti(s) + 2MgCl2(s) How many grams of TiCla(s) can be reduced to Ti by 2000.0g of Mg?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY