
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
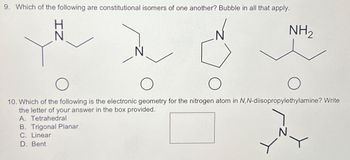
Transcribed Image Text:9. Which of the following are constitutional isomers of one another? Bubble in all that apply.
IZ
NH₂
10. Which of the following is the electronic geometry for the nitrogen atom in N,N-diisopropylethylamine? Write
the letter of your answer in the box provided.
A. Tetrahedral
B. Trigonal Planar
C. Linear
D. Bent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 35. Which of these is not an alkane? А. С.Н6 B. C¢H14 C. CAH10 D. C2H4 E. CH4arrow_forwardWhich of the following structures represents isopropyl methyl ether?arrow_forwarddraw the structure and give the systematic name of a compound with molecular formula C5H12 that a. only primary and secondary halogens b. only primary halogens c. one tertiary hydrogensarrow_forward
- An ester with the formula C8H1602 gives an alcohol and an acid when hydrolysed. When the alcohol is isolated and oxidized, it forms a ketone. Which of these formulas cannot be the ester? Xoy 4 Select one: OA. I OB. II OC. III O D. IV O E. V I O IV f r II V مر H III Oarrow_forwardChange 1,2-ethenediol into 1,2 ethynediol (HOCCOH) by removing one hydrogen atom from each carbon atom and replacing with a triple bond between the carbon atoms. Be sure to add the third rod to represent the triple bond. Draw a Lewis structure for 1,2-ethynediol. A. Identify the electron-pair and molecular geometry around each carbon and oxygen atom. B. What are the bond angles for C-C-O? For C-O-H? C. Can the molecule interconvert or “flip” between different structures in which the –OH group on the two carbon atoms occupy different orientations with respect to each other? If so, how does this happen? If not, why?arrow_forward2. Identify & Name the functional groups are in the following molecules: `NH2 а. `NH2 b. но N.arrow_forward
- There is one functional group (not counting alkanes, alkenes, cydic, alkynes, and aromatic) in the compound below. What functional group is CH3 CH3 Oa. ketone O b. ester Oc. ether O d. alcohol O e. aldehyde O Ai ere to searcharrow_forwardWhich of the following statements can be used to prove that carbon is tetrahedral? a. CH3Br does not have constitutional isomers. b. CBr4 does not have a dipole moment. c. CH2Br2 does not have constitutional isomers.arrow_forwardTo name carboxylic acids use the name of the pare “_ Oi C 0ic " at the end. Example: Name the following: H. .C. C C. HO H. H. 188 エ エー○ エIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY