
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
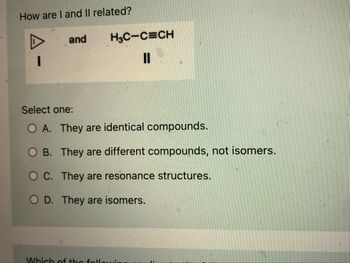
Transcribed Image Text:**How are I and II related?**
**Compound I**: Cyclopropane represented by a triangle.
**Compound II**: Chemical formula \(\text{H}_3\text{C-C}\equiv\text{C-H}\), an alkyne.
**Select one:**
- **A.** They are identical compounds.
- **B.** They are different compounds, not isomers.
- **C.** They are resonance structures.
- **D.** They are isomers.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Utilize the structure of Benadryl (Diphenhydramine) shown below to determine its molecular formula. OA.C17H21NO OB. C15H23NO C.C17H23NO OD. C17H11 NO N_arrow_forwardMacmillan Learni Each of the Lewis structures shown contains one polar covalent bond. Identify the bond, and use the 8+/8- notation to show the direction of polarity. H :0: .С. I H. OOO :NEC-H 8- Answer Bank 8+ DOD PIH H-P―CI:arrow_forwardQUESTION 11 OH О а. NaOH Ob. Ag20, H20, NH4ОН O C. BrH4 O d. CrОз, HC, руridine O e. CrO3, H20, H2SO4 Of. NABH4 Og. PH3PO O h. LIAIH4 O i. NaHarrow_forward
- Questions 6a, b, c, d is finked to one another and focus on the molecule shown below. O: H C2 H. I-Z:arrow_forwardJj.11.arrow_forwardREPRESENTATIONS OF ORGANIC MOLECULES Identifying isomers and resonance structures Determine the relationship between Structure A and Structure B in each row of the table. Structure A Structure B Relationship H. o isomers H. H H -H H-C- C-O -H- O resonance structures H H. H H Н—С—Н o neither H o isomers H :0: :0-H H. H H- C-C C-H H-C=C C=C-H O resonance structures H. H. H o neither o isomers :o: H H. H- O resonance structures H H H. H o neither 72°F DELL :ö: IIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY