
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
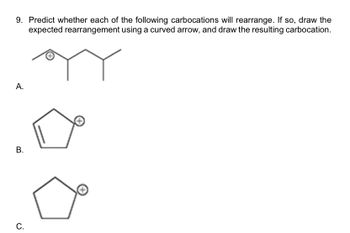
Transcribed Image Text:9. Predict whether each of the following carbocations will rearrange. If so, draw the
expected rearrangement using a curved arrow, and draw the resulting carbocation.
A.
B.
C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can this carbocation rearrange to a more stable one?arrow_forward15. a. Label the reactive features, highlight the most reactive one, then highlight what it needs. Also, state if the reaction will start to create a carbocation, carbon radical, or carbanion, or will cause loss of aromatic character. If a carbocation, carbon radical, or carbanion starts to develop, label where that will occur. (Z)-2-methylpenta-1,3-diene with HBr b. Use mechanism arrows to illustrate the reaction that occurs. c. If applicable, use stabilization resources to deal with the carbocation, carbon radical, or carbanion that starts to develop during the reaction, and draw the structure of any resonance-stabilized intermediate. d. Continue labeling and diagramming the reaction until you find the major stable product(s). e. Finally, state the stereochemistry of the major product(s) and use either Fisher projection or perspective formula representations to illustrate that stereochemistry.arrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. What is the most stable carbocation below?arrow_forward
- A. Predict the major product of the reaction. Clearly indicate stereochemistry, if necessary. 1. NaNH2 (excess) NH3/THF Br Br i-Pr 2. H3O* B. Draw a detailed arrow-pushing mechanism for the transformation, accounting for stereochemistry, if necessary.arrow_forwardIf reacted with Cl2 how many distinct mini chlorinated products will be present? How many monocholtinated products will form at 1 and 2? Which Carbon will be attached to a Cl in the major product?arrow_forwardFor each of the series of electron-pushing arrows, please draw the respective products. Also, indicate if each reaction is an example of initiation, propagation, or termination.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY