
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
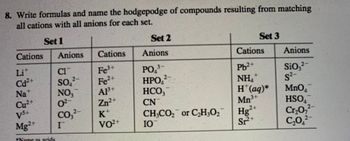
Transcribed Image Text:8. Write formulas and name the hodgepodge of compounds resulting from matching
all cations with all anions for each set.
Set 1
Cations Anions
Li
Cd²+
Na
Cu²+
Vs+
Mg2+
*Name is veids
CI
SO₁²
NO,
0²
CO3²-
I
Cations
Fe³+
Fe²+
A1³+
Zn²+
K*
VO²+
Set 2
Anions
3
PO ³
HPO,²
HCO,
CN
CH₂CO₂ or C₂H₂O₂
IO
MEDA
Set 3
Cations
Pb²+
NH₂
H*(aq)*
Mn³+
Hg2+
Sr²+
Anions
SiO₂²
S²-
MnO₂™
HSO
A
Cr₂O7²
C₂0,2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following substances are largely ionic, and which are covalent? Drag the appropriate items to their respective bins. Reset Help HI NaOH CH;Li BBr3 HF PdCl, lonic Polar covalent Nonpolar covalentarrow_forwardChoose the answer that correctly fills the blanks in the following sentence: Based on the octet rule, the element aluminum will likely form a ______ charge because it will ______. Group of answer choices 3+; lose 3 electrons Aluminum does not form an ion. 3-; gain 3 electrons 5-; gain 5 electrons 3+; gain 3 electronsarrow_forwardUsing just a periodic table (not a table of electronegativities), decide which of these is likely to be the most polar bond. 3A 4A 5A 6A 7A 5 6 7 8 artially Correct O O O O 9 BCNO F 13 17 14 15 16 Al Si P S CI 31 32 33 34 35 Ga Ge As Se Br 49 50 51 52 53 In Sn Sb Te I P-O 82 83 84 85 11 Pb Bi Po At Si-O N-O S-Oarrow_forward
- Profiles Tab Window Help Paraph cics....pdf Gramm nment/takeCovalent Activity.do?locator=assignment-take Su My Ho OV X A Common Greek(upper) X⁰ XX Undo Redo Clear Help The compound ZnSO3 is an ionic compound. What are the ions of which it is composed? Use the References to access important values if needed for this question. World view.pdf 14 How to tv Cation formula DIC Greek(lower) Works G Comp Anion formula Arrows Other Aa (s) (1) (g) (aq) ali C Cheg u ining Chem + ★河口 Previous Next Show All A X сarrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure esc H= Br ! : 0: 1 Cl Explanation Q A :0: || g N The symbols of the problem atoms are:* 0 * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,O". : 0: 2 Cl: Check W S Yes. O No, it has the wrong number of valence electrons. The correct number is: O No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 O Yes. O No, it has the wrong number of valence electrons. The correct number is: O No, it has the right number of valence electrons but doesn't satisfy the octet rule. 0 Is the proposed Lewis structure reasonable? The symbols of the problem atoms are:* Yes. O No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons…arrow_forwardFor each bond, show the direction of polarity by selecting the correct partial charges.arrow_forward
- Fill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead. molecular formula BP C₂S₂ CS₂ BI3 H₂O name of compound X П 0 0 0 Śarrow_forward5. Complete the table below. Formula Valence electrons Total number of Lewis Dot for each atom valence electron Structure NOF C3H5 H30*1 CN-1arrow_forwardFind the covalent compound from the given choices. O K2O Na,0 O Li¿Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY