
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
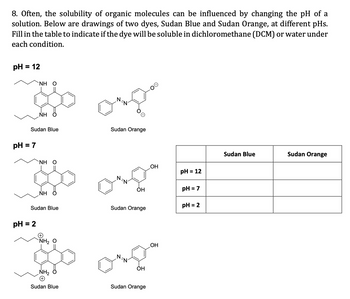
Transcribed Image Text:8. Often, the solubility of organic molecules can be influenced by changing the pH of a
solution. Below are drawings of two dyes, Sudan Blue and Sudan Orange, at different pHs.
Fill in the table to indicate if the dye will be soluble in dichloromethane (DCM) or water under
each condition.
pH = 12
pH = 7
`ΝΗ Ο
Sudan Blue
ΝΗ
pH = 2
`ΝΗ Ο
ΝΗ
Sudan Blue
NH, O
NH₂
Sudan Blue
Sudan Orange
OH
Sudan Orange
OH
Sudan Orange
OH
OH
pH = 12
pH = 7
pH = 2
Sudan Blue
Sudan Orange
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp compound BaCO3 NaBr KC1 Does solubility change with pH? yes no yes no yes no pH = 7 highest solubility pH = 6 X pH = 5arrow_forwardThe ionization constant of a very weak acid, HA, is 1.9x10-9. Calculate the equilibrium concentrations of H30", A", and HA in a 0.040 M solution of the acid. Determine the concentrations of all species at equilibrium and the solution pH. [H3O"] =| mol/L [A'] =[ mol L [HA] = | mol L pH =arrow_forwardFor the titration of 70.0 mL of 0.400 M NH, with 0.500 M HCl at 25 °C, determine the relative pH at each of these points. Determine the relative pH before the addition of any HCl. pH > 7 O pH 7 Determine the relative pH after 71.0 mL HCl has been added. O pH = 7 pH > 7 O pH < 7arrow_forward
- In general chemistry we taught you to calculate the pH of a solution if you know the H30+ concentration. We neglected to talk about the concept of "activity"; when other ions in solution affect the behavior of the your analyte (analytical target) ion. This behavior is what we refer to as the ion activity. Please note that this only applies to ions - charged species - not uncharged molecules. Using the chart of activity versus ionic strength found in chapter 8 (7th edition) of the text, answer the following for a solution that has the following concentrations: [HCI] = 0.0118 and [KNO3] = 0.017 1) - the activity coefficient of H30+ ions = 2) - the activity of H30+ ions 3) - neglecting activities the pH of the solution is = 3) - accounting for activities the pH of the solution is =arrow_forward2. Given the H3PO4 - NaH₂PO4 buffer system, which of the following is false when KOH is added into the said solution? O The concentration of the H3PO4 will decrease upon addition of KOH. O The concentration of the H₂PO4 will decrease upon addition of KOH. O The concentration of the H₂PO4 will increase upon addition of KOH. O The [H₂PO4 ]/[H3PO4] will decrease due to the neutralization of the added KOH.arrow_forwardA buffered solution containing dissolved aniline, CH,NH,, and aniline hydrochloride, CH, NH, Cl, has a pH of 5.27. Determine the concentration of CH, NH in the solution if the concentration of CH, NH, is 0.275 M. The pK, of aniline is $ 9.13. [CH_NH] = = Calculate the change in pH of the solution, ApH, if 0.386 g NaOH is added to the buffer for a final volume of 1.35 L. Assume that any contribution of NaOH to the volume is negligible. ApH = Marrow_forward
- For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find Ksp data in the ALEKS Data tab. compound Does solubility change with highest solubility pH? pH = 6 pH = 4 pH = 3 ○ yes CaBr2 Ba(OH)2 yes no no CaCl₂ yes ○ no X Sarrow_forwardYou are on one of Saturn's many moons and discover a substance, Y2O that undergoes autoionization just like water on Earth. Y2O + Y2O ⇌ Y3O+ + OY– Through a series of experiments, you determine the equilibrium constant (Keq) for this autoionization reaction at various temperatures. The value of Keq at 28.7ºC is 7.6 x 10-14. What is the pY of the pure substance at this temperature? Report your answer to the hundreths placearrow_forwardFill out the tables and find pHarrow_forward
- 5) Find the concentration of H30*(aq) in a 1.75 M solution of lactic acid, HC3H5O3, at 25°C. Ka= 1.38 x 10*. 6) Write the equilibrium expression for the ionization of HOI, and calculate the concentration of HOI(aq) in solution if [H3O*]=2.3 x 10° M and pKa = 10.7 at 25°C.arrow_forwardPLease help me asap and make sure your answer is correct and rounded only at the final answer !arrow_forward50.0 mL of 0.100 M hydrochloric acid moles of H+ is equal to 0.005 100.0 mL of 0.200 M nitric acid moles of H+ is equal to 0.02 500.0 mL of 0.0100 M barium hydroxide moles of OH- is equal to 0.01 200.0 mL of 0.100 M rubidium hydroxide moles of OH- is equal to 0.02 When it asks you calculated the four moles of the solutions, this is what they are referring to.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY