
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
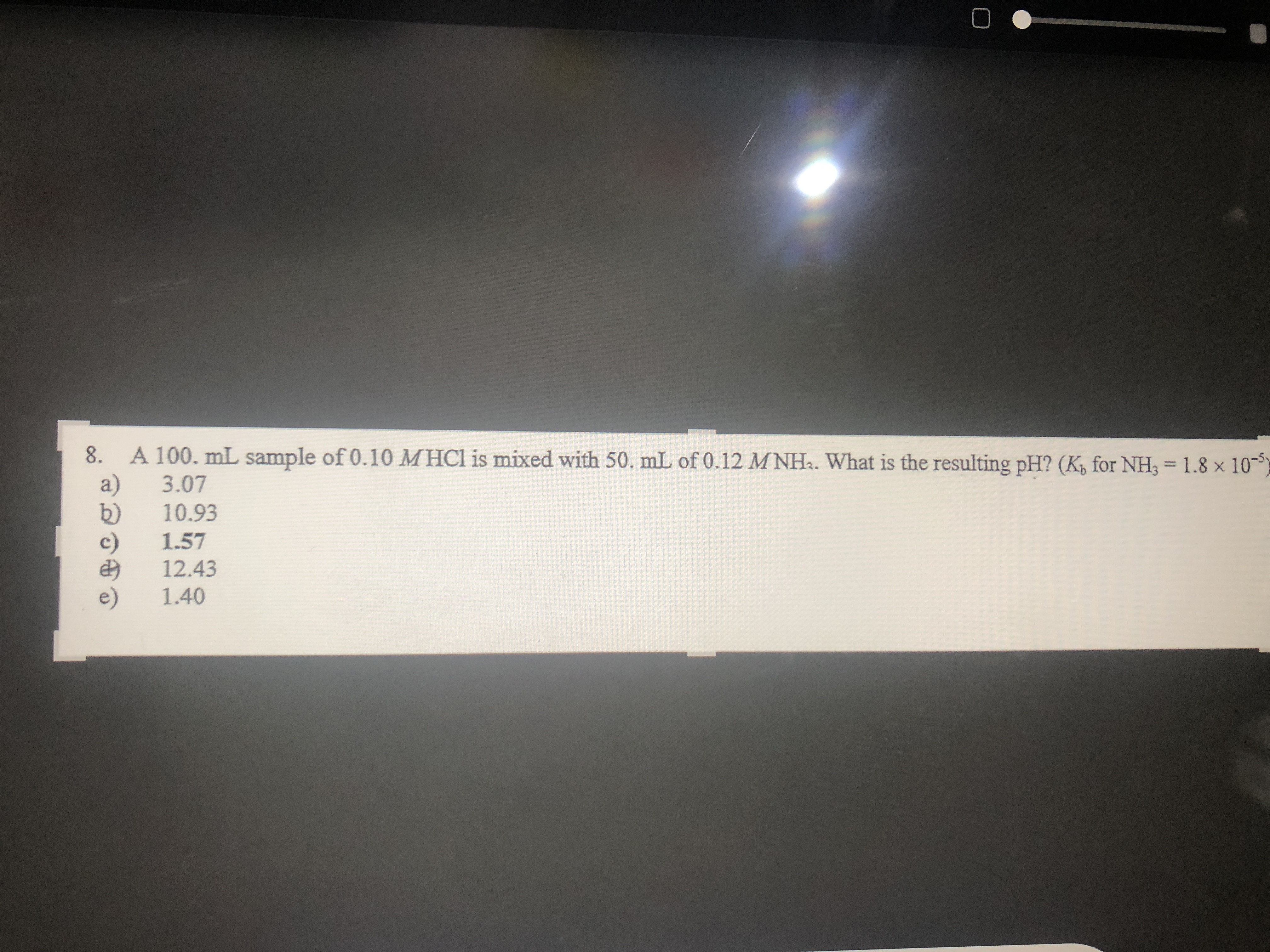
Transcribed Image Text:8.
A 100. mL sample of 0.10 MHCI is mixed with 50. mL of 0.12 M NH.. What is the resulting pH? (K, for NH3 = 1.8 × 10--
3.07
10.93
1.57
12.43
1.40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pH of a buffer solution that is 0.416 M in ammonia and 0.406 M in ammonium chloride (NH4CI)? The K, of ammonia is 1.8 x 10-5. (Two decimal places) Type your answer...arrow_forwardAll one question plz anwser neatly? thanks ?arrow_forwardA 39.6 mL sample of a 0.270 M solution of NaCN is titrated by 0.330 M HCl. Kb for is CN- is 2.0x10^-5 calculate the pH at: 1. at the equivalence point 2. after the addition of 39.9 mL of 0.330 M HClarrow_forward
- A 10.0 ml solution of 0.330 M NH, is titrated with a 0.110 M HCl solution. Calculate the pH after the following additions of the HCI solution: 0.00 ml. 10.0 ml., 30.0 ml., and 40.0 ml.. Round your answer to 3 decimal places. Note: Reference the X, of acids at 25 °C and K, of bases at 25°C tables for additional information. Part 1 of 4 tharrow_forward1. A 0.36 M hydrocyanic acid (HCN) solution has a pH of 4.87. Give: (a) the balanced equation for the ionization of hydrocyanic acid; (b) K. of HCN; (c) % ionization of HCN. a. b.arrow_forwardThe 100. mL of buffer solution is 0.05 M in C2H3O2- and 0.10 M in HC2H302. What is the resulting pH when 20.0 mL of 0.1 M HCl is added to the buffer? Ka of HC2H302 = 1.8x10-5 pH after strong acid added = [?] pH of Soln w/ Acid Enterarrow_forward
- When a solution is titrated with an base, an indicator (HIn) changes from yellow to blue (In). The Ka of the indicator is 8.00e - 06. At what pH will the indicator color change first be visible? pH = 6.0969 X 0%arrow_forward5:48 PM Wed Nov 16 Today 5:48 PM 目。 oint when 15.0 ml. of o. 75% Calculate the pH of a titration at the point when 15.0 mL of 0.15 M NaOH is added to 30.0 mL of 0.20 M HNO3 Edit G ¯¯¯arrow_forwardA buffer solution is prepared that is 0.78 M HCN and 0.14 M NaCN. What is the pH of the solution? (Ka(HCN) = 4.9 × 10−10) 4 sig figs pleasearrow_forward
- Given: 0.300M NaOH 35.0L of 0.450M CH3COOH solution. CH3COOH dissociate constant = 1.8*10^-5 write out the equilibrium equation formed at the equivalence point.arrow_forwardCalculate the pH when 4.0 mL of 2.0 M NaOH is added to the buffer solution containing 25mL of 1.0 M HC,H;O2 and 25mL of 1.0 M NaC2H;O2. Where, K, = 1.8m10-5. 4.75 5.03 4.50 5.25arrow_forwardA 31.1 mL sample of a 0.190 M solution of NaCN is titrated by 0.230 M HCl. Kb for is CN- is 2.0x10^-5 Calculate the pH of the solution: 1. after the addition of 35.7 mL of 0.230 M HClarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY