
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

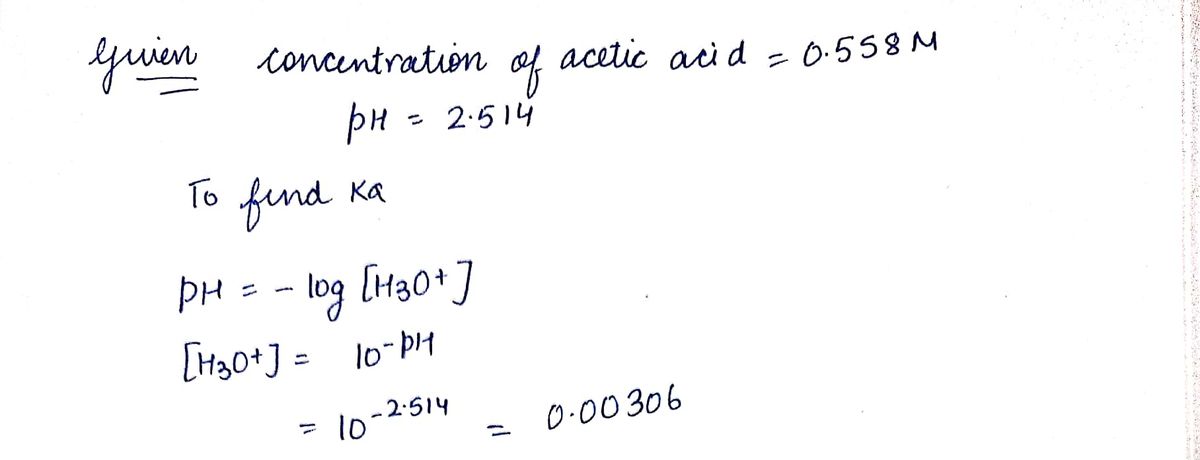
Transcribed Image Text:a the laboratory, a general chemistry student measured the pH of a 0.558 M aqueous solution of acetic acid to be 2.514.
se the information she obtained to determine the K, for this acid.
a(experiment) =
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The pH of an aqueous solution with [OH-] of 2.45×10-8 M is ……………….. and the solution is……………….arrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.319 M aqueous solution of codeine, C13H210;N to be 10.742. Use the information she obtained to determine the K, for this base. K„(experiment)arrow_forwardPlease don't provide handwritten solution ....arrow_forward
- In the laboratory, a general chemistry student measured the pH of a 0.359 M aqueous solution of aniline, C6H5NH2 to be 9.228. Use the information she obtained to determine the K, for this base. Kblexperiment) = Submit Answer Retry Entire Group 9 more aroup attempts remainingarrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.483 M aqueous solution of triethylamine, (C2H5)3N to be 12.129. Use the information she obtained to determine the K, for this base. Kb(experiment) : %3Darrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.558 M aqueous solution of formic acid, HCOOH to be 1.984. e information she obtained to determine the K, for this acid.arrow_forward
- Pls ans botharrow_forwardJA chemist makes a 1.0 L of a solution that is both 0.6 M NH, and 0.8 M NH,CI. pK, of NH, = 4.75. What is the pH of this solution? Round your answer to two decimal places. pH =arrow_forwardOne solution contains 0.150 MHA (weak acid) and 0.250 M NaA (weak conjugate base).. The K, value for the weak acid is K, 3.0x 10 6. Determine the pH of the solution. Type your answer in the space below.arrow_forward
- In the laboratory, a general chemistry student measured the pH of a 0.597 M aqueous solution of hydroxylamine, NH2OH to be 9.880. Use the information she obtained to determine the K, for this base. Куехрeriment)arrow_forwardInitial pH: 1.55 Molarity: 0.081158arrow_forwardAll one question plz anwser neatly? thanks ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY