
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
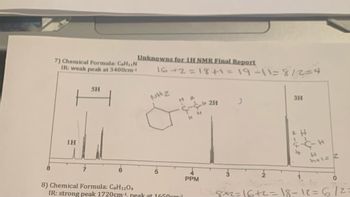
Transcribed Image Text:**Unknowns for 1H NMR Final Report**
**7) Chemical Formula: C₆H₁₁N**
- **IR Spectroscopy:** Weak peak at 3400 cm⁻¹.
**NMR Spectrum Analysis:**
- **Graph Description:**
- The spectrum shows peaks at different parts per million (PPM).
- A peak at approximately 7.5 PPM indicates the presence of aromatic protons (labeled as 5H).
- A peak at approximately 3.5 PPM indicates the presence of 2 protons adjacent to an amine group (NH₂). A hand-drawn structure suggests a CH₃-CH₂-NH₂ group.
- A peak at approximately 1.2 PPM represents 3 aliphatic protons, typically indicative of a methyl group.
**Handwritten Calculations and Notes:**
- The equation "16 + 2 = 18 + 1 = 19 - 11 = 8 / 2 = 4" suggests a step-by-step mass balance or hydrogen count aligned with the spectrum interpretation.
**8) Chemical Formula: C₈H₁₂O₄**
- **IR Spectroscopy:** Strong peak at 1720 cm⁻¹, peak at 1650 cm⁻¹.
**Handwritten Calculation:**
- The note "8 x 2 = 16 + 2 = 18 - 12 = 6 / 2 = 3" possibly relates to structural deductions or hydrogen equivalence.
This analysis provides insights into the structural determination of the unknown compound using 1H NMR spectroscopy alongside IR data.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please confirm if this H-NMR spectrum belongs to this molecule and identify the signals of each spectrum.arrow_forwardanswer the following questions about the 1-Butanol-,3-methyl-acetate H NMR 1- identify the number of the chemical shift of peaks, signals, and integration of the NMR 2-Justify the identity of the compound using the NMR (how does this NMR represent 1-Butanol-,3-methyl-acetate?)arrow_forwardHow many signal(s) will appear in the H-NMR spectrum of acetone (structure shown below)? 4 O 1arrow_forward
- #3) Using the following spectra and other information provided, identify the compound. Assign pertinent peaks in the spectra. Label the peaks on the spectrum and place the structure of the compound in the box on the right side of the spectrum - Identify correct compound - label proton spectrum - label carbon spectrumarrow_forward#7) Using the following spectra and other information provided, identify the compound. Assign pertinent peaks in the spectra. Label the peaks on the spectrum and place the structure of the compound in the box on the right side of the spectrum - Identify correct compound - label proton spectrum - label carbon spectrumarrow_forwardUnknowns for 1H NMR Final Report 10) Chemical Formula: C₁₂H1s (hard to see but, all peaks that integrate to 1H are doublet of doublets) IR: peak at 1650cm-1 2H 10 2H 1H u 7 1H 11) Chemical Formula: C₁0H12O2 IR: strong peak 1720cm-¹ 6 1H 1H 1H 8 12) Chemical Formula: C₂H₂00 IR: strong broad 3300cm-1 5 2H 2H 1H 3 4 PPM 6 PPM -3 septet 1H PPM 9H 6H 18H 0arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY