
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
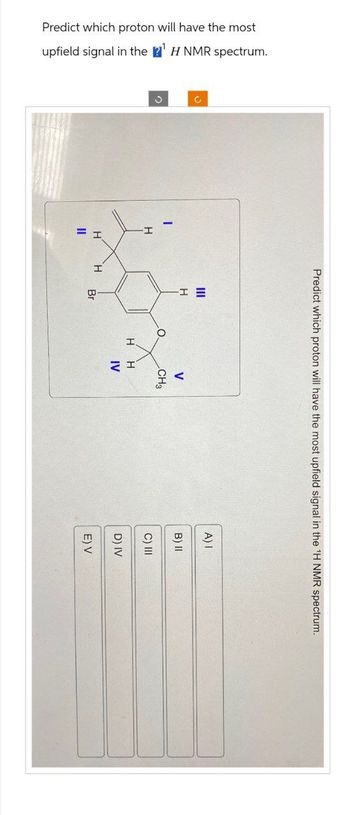
Transcribed Image Text:Predict which proton will have the most
upfield signal in the ¹ H NMR spectrum.
O
H
Predict which proton will have the most upfield signal in the ¹H NMR spectrum.
H H
11
H
Br
O
H
V
CH3
H
IV
A) I
B) II
C) III
D) IV
E) V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the following spectra. Please find peaks are assigned on the spectrum (~5-6 peaks). Label the peaks on the spectrum and place the structure of the compound in the box on the lower left-hand corner of the spectrum (from the table below, no numbering scheme). You do not have to indicate the exact wavenumber of the peak.arrow_forward5, Draw the correct molecule in the box to the right. Label the relevant peaks in the spectra with the proper functional group. If the absence of key peaks led to your choice, indicate which key peaks were absent.arrow_forwardWhich of the following will show exactly two peaks in its normal broadband proton decoupled 13C NMR?arrow_forward
- v Instructions Question 18 What is the correct assignment in the number of peaks in the H NMR spectra of th Br Br Br Br Br Br II II O I = 3 signals; II = 2 signals; III = 3 signals. O I = 4 signals; II = 4 signals; III = 4 signals. O I = 2 signals; II = 3 signals; III = 1 signals. O I = 3 signals; II = 3 signals: III = 2 signals. • Previousarrow_forward3, Draw the correct molecule in the box to the right. Label the relevant peaks in the spectra with the proper functional group. If the absence of key peaks led to your choice, indicate which key peaks were absent.arrow_forwardFor the dimedone molecule, how many different signals would you see in the proton NMR? Assume you can see them allarrow_forward
- The intensity of a signal in a ¹H NMR spectrum is determined by O Number of non-equivalent protons O Number of diasteriotiotopic carbons O The number of neighboring protons O Number of enantiotopic protons O The electronic environment of protons O Number of equivalent protons apie genann OPB88888 Witaarrow_forwardQ1arrow_forwardRank the signals of the following compound in terms of increasing chemical shift. Identify the proton(s) giving rise to each signal. Show your work! H OHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY