
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
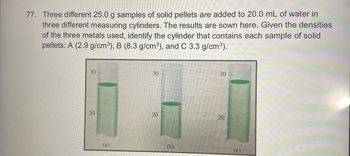
Transcribed Image Text:77. Three different 25.0 g samples of solid pellets are added to 20.0 mL of water in
three different measuring cylinders. The results are sown here. Given the densities
of the three metals used, identify the cylinder that contains each sample of solid
pellets: A (2.9 g/cm³), B (8.3 g/cm³), and C 3.3 g/cm³).
30
20
(a)
30
20
(b)
30
20
(c)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A soft drink contains 54 gg of sugar in 339 gg of H2OH2O. Part A What is the concentration of sugar in the soft drink in mass percent? Express your answer in percent to two significant figures.arrow_forwardRemaining Time! 02:29:4. Consider the balanced chemical equations shown below. When a 6.00 g sample of a mixture of iron (Fe) and aluminum (Al) is treated with excess HCl(aq), 0.177 moles of H2 are obtained. What is the mass percentage of Fe in the original mixture? Choose the closest answer. Fe(s) + 2 HCI(aq) FeCl2(aq) - + H2(g) 2 Al(s) +6 HC((aq) 2 AICI3(aq) + 3 H2(g) 96 % 69 % 47 % 33 % 17 % Rack Question Menu - O Oarrow_forwardDetermine the volume of 0.240 MM KOHKOH solution required to neutralize each sample of sulfuric acid. The neutralization reaction is:H2SO4(aq)+2KOH(aq)→H2SO4(aq)+2KOH(aq)→ K2SO4(aq)+2H2O(l) 160 mL of 0.155 M H2SO4 Express your answer using three significant figures.arrow_forward
- An acid, HEl, is formed from a hypothetical element, El, and a subsequent aqueous solution is created with the acid and water. The solution is measured to be 22.01% HEl by weight. Calculate the mol % of the acid in the solution. Atomic Mass: H: 1.00784 g/mol O: 15.999 g/mol El: 160.33996 g/mol Round your answer to 2 decimal places.arrow_forwardReport your answer to 3 significant figures in scientific notation (example: 1.23E+4) unless stated otherwise. DO NOT include the units in your answer. 1. Consider a reaction of 827 mL of a 6.14×10-2 M K,S04 solution completely reacts with 131 mL of a 3.54x102 M CaI, solution according to the following UNBALANCED chemical equation. K2SO4(aq) + Cal2(aq) → CaSO4(s) + KI(aq) (a) Balance the chemical equation using the lowest integer coefficients. Be sure to include all coefficients including those of 1. 1 K2SO4(aq) + 1 Cal2(aq) 1 CaSO4(s) + 2 KI(aq) You are correct. Your receipt no. is 155-6200 ? Previous Tries (b) Determine the mass (in g) of CaSO4 that is formed in the reaction. Report your answer to 3 significant figures in standard notation (example: 1.23). g Submit Answer Tries 0/99 (c) If 3.58x10-1 g of CaSO4 is produced, what is the percent yield of the reaction? Report your answer to 3 significant figures in standard notation (example: 1.23).arrow_forward90 80 NH.CI 70 HCI 60 KCI 50 40 NaCI -KCIO, 30 20 NH3 10 10 20 30 40 50 60 70 80 90 100 Temperature (°C) You have a solution containing 50.0 grams of KNO3 dissolved in 100.0 g of water at a temperature of 50.0°C. If you decided to add 20.0 more grams of KNO3, what would happen? O None of the additional 20 grams of solute would dissolve. All of the additional 20 grams would dissolve. Only a few of the 20 additional 20 grams of solute could be dissolved. O A lower temperature would be required to dissolve all 20 of the additional grams of solute Solute per 100g of Harrow_forward
- Sample 1 Sample 2 Sample 3 Mass of Erlenmeyer Flask (g) 24.33 24.37 24.44 Mass of Erlenmeyer Flask + Calcium Hydroxide Solution (g) (g)(lime water) 27.22 27.29 27.33 Mass of Calcium Hydroxide Solution (g) 2.89 2.92 2.89 Volume of Ca(OH)2 Density = 1.000 g/mL 2.89 2.92 2.89 Concentration of HCl (M) 0.1 0.1 0.1 Initial HCl Volume in Syringe (mL) 1.00+1.00 1.00+1.00 1.00+1.00 Final HCl Volume in Syringe (mL) .72 .66 .54 Volume of HCl Delivered (mL) 1.28 1.34 1.46 Moles of HCl Delivered Moles of OH- in Sample Moles of Ca2+ in Sample Molar Solubility (M) Calculated Ksp Average Calculated Ksp I am very confused how to solve the blank spots in the table. Everything including and after "Moles of HCl Delivered" confuses me. Please help me figure out how to solve the sections correctly. Also feel free to…arrow_forwardHow many moles of Mg(ClO2)2 are present in 60.0 mL of 1.00 M Mg(ClO2)2 solution? Note: Answers must be numeric, not alphanumeric—42, not forty-two. Do NOT express your answer in scientific form. KEY is supplied in decimal form only: — XXX.xxxx not X.X x 102arrow_forward4. A solution is prepared by taking a clean and dry 50.00 mL volumetric flask. Some sucrose (Mm 342.2 g/mol) is added to the flask with the following mass measurements recorded: mass of empty volumetric flask: 142.88 g mass of weighing dish + sucrose: 29.83 g mass of weighing dish after adding sucrose to volumetric flask: 24.62 g Some water is added to the flask, the solution stirred until all the sucrose is dissolved. Then water is added carefully to the mark on the flask. The filled flask is then weighed again. mass of filled volumetric flask: 187.30 g What is the mole fraction of water? Answer to 3 decimal places.arrow_forward
- Please use the corrrect significant digits (3), if needed use scientific notation.arrow_forwardNo ai use okkkarrow_forwardAn aqueous solution at 25 °C has a H₂O* concentration of 7. x 10-6 M. Calculate the OH concentration. Be sure your answer has the correct number of significant digits. M 0 10 X Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY