
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN: 9781305387102
Author: Kreith, Frank; Manglik, Raj M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
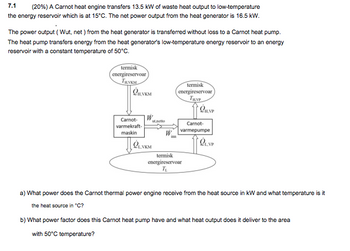
Transcribed Image Text:7.1
(20%) A Carnot heat engine transfers 13.5 kW of waste heat output to low-temperature
the energy reservoir which is at 15°C. The net power output from the heat generator is 16.5 kW.
The power output (Wut, net) from the heat generator is transferred without loss to a Carnot heat pump.
The heat pump transfers energy from the heat generator's low-temperature energy reservoir to an energy
reservoir with a constant temperature of 50°C.
termisk
energireservoar
Онукм
Carnot-
varmekraft-
maskin
LVKM
шлепо
termisk
termisk
energireservoar
TRYP
QH.NP
Carnot-
varmepumpe
QLVP
energireservoar
T₁
a) What power does the Carnot thermal power engine receive from the heat source in kW and what temperature is it
the heat source in "C?
b) What power factor does this Carnot heat pump have and what heat output does it deliver to the area
with 50°C temperature?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Refrigerators currently being manufactured in the United States are using______as their refrigerant.arrow_forwardThe operating condition for the single compressor in a household refrigerator is the lowest box temperature, which is typically A. 0F B. -20F C. 20F D. 40Farrow_forwardB) A reversible heat engine operates between two reservoirs at temperatures of 600°C and 40°C. The engine drives a reversible refrigerator which operates between reservoirs at temperatures of 40°C and -20°C. The heat transfer to the heat engine is 2000 kJ and the net work output for the combined engine refrigerator is 360 kJ. (i) Calculate the heat transfer to the refrigerant and the net heat transfer to the reservoir at 40°C. (ii) Reconsider (i) given that the efficiency of the heat engine and the C.O.P. of the refrigerator are each 40 per cent of their maximum possible values.arrow_forward
- A heat engine operates between two reservoirs at 800 and 20oC. Onehalf of the work output of the heat engine is used to drive a Carnot heatpump that removes heat from the cold surrounding at 2oC and transfersit to a house maintained at 22oC.If the house is losing heat at a rate of 62,000 kJ/h,Determine:a) The minimum rate of heat supply (kJ/h) to the heat enginerequired to keep the house at 22oC. b) The thermal efficiency of the heat engine, if the rate of heatsupply to the heat engine is 15,000 kJ/h. c) The total amount of entropy change of the surrounding by takinginto account both the heat engine and Carnot heat pump for the casesof i) and ii), respectively. d) The cyclic entropy change of the heat engine for the cases of i) andii), respectively.arrow_forwardA heat pump operates with a Coefficient of Performance equal to 8. What is the COP if this cycle were to operate as a refrigerator?arrow_forwardA parameter used to characterize the heat pump cycle performance is called the thermal efficiency. True or False?arrow_forward
- True or false Heat is transferred to a system by either a cheap commercially available air-sourced heat pump with coefficient of performance of just 1.5, or an expensive 100% efficient electrical resistance heater. The loss of Carnot Work Potential (Exergy destruction, or T0Sloss term) is minimum when heat is added by the cheap commercial heat pump. Heat is transferred to a system by either a cheap commercially available air-sourced heat pump with coefficient of performance of just 1.5, or an expensive 100% efficient isothermal heat source. The loss of Carnot Work Potential (Exergy destruction, or T0Sloss term) is minimum when heat is added by the isothermal heat source.arrow_forwardA heat pump absorbs heat from the cold outdoors at 2° C and supplies heat to a house at 23° C at a rate of 36,000 kJ/hr. What is the COP of the heat pump if the power consumed is 1.7 kW? b. What is the maximum COP of the heat pump? а.arrow_forwardA homeowner is trying to decide whether to heat with a furnace rated at 95% efficiency or by an electrically powered heat pump. She lives in a town where electricity is produced by a coal-fired power plant that claims to operate with 1st law efficiency that is 55% of the Carnot limit. The heat pump's CoP is advertised to be 40% of the Carnot limit. For what range of outside temperatures To would the 2nd law efficiency of the furnace be greater than that of the heat pump? Assume T+ = 400°C, T- = T= 40°C. %3Darrow_forward
- Please I need solutions to this questionsarrow_forwardData are provided for two reversible refrigeration cycles. One cycle operates between hot and cold reservoirs at 27°C and 3°C, respectively. The other cycle operates between the same hot reservoir at 27°C and a cold reservoir at -35°C. The refrigerator removes the same amount of energy by heat transfer from its cold reservoir. Determine the ratio of the net work input values of the two cycles, WCycle,2 Wcycle,1arrow_forwardA heat engine with a thermal efficency of 45% rejects 500kJ/kg of heat. How much heat does it recieve?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

Principles of Heat Transfer (Activate Learning wi...
Mechanical Engineering
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Cengage Learning

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning