
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Can you please help me problem # 7 and explain to me the correct answer?
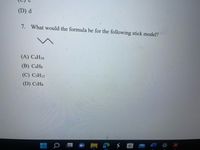
Transcribed Image Text:(D) d
7. What would the formula be for the following stick model?
(A) C4H10
(B) САНS
(C) CSH12
(D) C3H3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A small business brings in a revenue of $60000 for the week. The payroll for the week is $20000. The owner spends $1000 on the company anniversary party. Of what is left, one third is donated to a local charity, and $500 less than half the amount that went to the charity goes to building repairs. What is the final amount of profit for the week? Question 5 options: $20000 $7000 $13000arrow_forwardG1arrow_forwardMy experiment was to have eight drops of liquid in each well. For a one I had five drops of Na2S2O3 and three drops of hydrochloric acid. For well A2 I had four drops of Na2S2O3, One drop of distilled water, and three drops of hydrochloric acid. For well a three I had three drops of Na2S2O3, two drops of distilled water, and three drops of hydrochloric acid. Four well a four I had two drops of Na2S2O3, three drops of distilled water, and three drops of hydrochloric acid. Use the table for information to answer the questions below. Additional information: hydrochloric acid = 0.1M and the sodium Thiosulfate (Na2S2O3) = 0.1M. I already have the information for 1-3 including the table filled out and graph (included if needed). The main questions I need help with is #4-6arrow_forward
- Ksp =arrow_forwardConstruct the expression for Kc for the following reaction. 1 3NO(g) = N20(g) + NO2(g) Construct the expression for Kc for the following reaction. 2 X 4HCI(aq) + O2(g) = 2H20(1) + 2C12(g) > Construct the expression for Kc for the following reaction. 3 CH4(g) + 2H2S(g) = CS2(g) + 4H2(g) Construct the expression for Kc for the following reaction. 4 X 2Cu*(aq) + Zn(s) = 2Cu(s) + Zn²*(aq) >arrow_forwardJust #2 pleasearrow_forward
- What is the molarity of C₁2H22O11 in an aqueous solution that is prepared by dissolving a 29.87 g sample of impure C12H22011 in water to make 725.0 mL of solution. Assume that the sample is 83.0% C12H22011 by mass. Enter your answer in mol L-1, accurate to three significant figures. Do not include units as part of your answer. Use the following molar masses, in g mol-¹, for your calculations. H, 1.008 Number C, 12.01 0, 16.00arrow_forwardDraw the structural formula of the product of the reaction shown below. Please draw a clear and detailed image.arrow_forwardRsimi, Nour 2023VVA Chemistry.7b.FA1 An unknown solid substance is heated according to the temperature versus time graph below. Temperature (°C) 70 40 30 20 288 8 2 0 0 5 15 Time (min) 20 00 4 of 5 1 2 3 4 5 25 While the substance is a liquid, at what temperature do the molecules of the substance have the greatest average kinetic energy? 2 % 3 4 5 16 7 8 insertarrow_forward
- 7B.arrow_forwardPlease give information about these chemicals.arrow_forwardacid A student prepares a 1.2 M aqueous solution of benzoic acid (CH,CO,H). Calculate the fraction of benzoic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY