
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
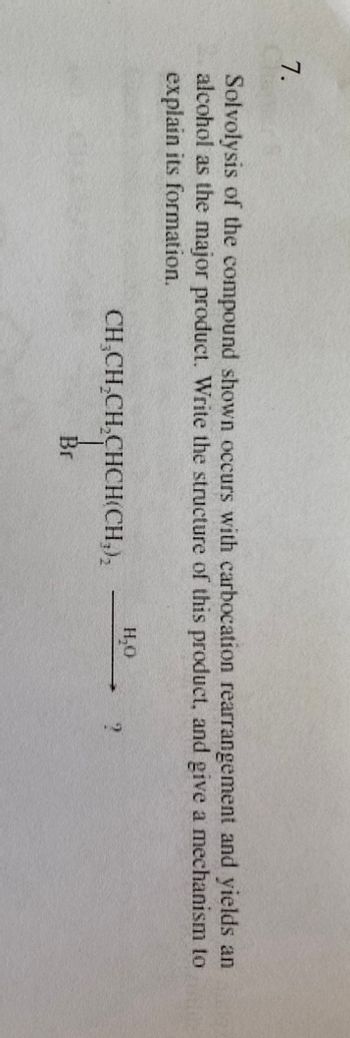
Transcribed Image Text:7.
Solvolysis of the compound shown occurs with carbocation rearrangement and yields an ex
alcohol as the major product. Write the structure of this product, and give a mechanism to
explain its formation.
CH₂CH₂CH₂CHCH(CH₂)₂
Br
H₂O
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
is there any line structure explanation of this?
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
is there any line structure explanation of this?
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In this chapter, we studied the mechanism of the acid-catalyzed hydration of an alkene. The reverse of this reaction is the acid-catalyzed dehydration of an alcohol. OH H,SO, CH,-CH-CH, CH-CH=CH, + H,O 2-Propanol (Isopropyl alcohol) Propene Propose a mechanism for the acid-catalyzed dehydration of 2-propanol to propene.arrow_forwardThe southern pine beetle utilizes a multi-component aggregation pheromone (one component shown below) to start mass colonization of healthy trees. The biosynthetic pathway involves the cyclization of this acetal from the straight chain structure. Draw the straight chain structure that could be used to form this acetal. Use wedges and dashes to correctly depict the stereochemistry. H3C- OH ✔arrow_forward5. There are three constitutional isomers with the molecular formula, C6H12. When heated with chlorine (Cl₂) at 300C, ● Isomer A gives a mixture of four monochlorination products. Isomer B gives a mixture of three monochlorination products. Isomer B gives only one monochlorination product. From this information, deduce the structures of isomers A, B, and C. Draw the products upon chlorination of each to explain your answer on the back of this sheet..arrow_forward
- Draw a structural formula for the major organic product of each reaction and specify the most likely mechanism by which each is formed.arrow_forwardGive the product(s) for the following reaction, predicting the major product, anc indicating by which mechanism each is formed. Br MeOH heatarrow_forwardWhich compound is a tertiary alcohol that may not be oxidized by H2CrO4 or MnO2? Which compound is a secondary alcohol that may be oxidized to a ketone by MnO2? Which compound is an alcohol that may be oxidized to an aldehyde by PCC?arrow_forward
- What is the NAME of the Mechanism for this reaction?arrow_forward7A Write the possible products of the following reactions, the mechanism by which they were formed. mentioning Br CH3OH CH3ONaarrow_forwardPredict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product can be formed, rank them in terms of their predicted stability (1 is most stable isomer, 2 the next most stable isomer, etc...) a) b) HD HD H CH3 ''CI H CH3 Br 'Br KOH EtOH KOH EtOH KOH EtOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY