
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For each of the following pairs of substances, predict which member of the pair will have the higher melting point, and indicate why.
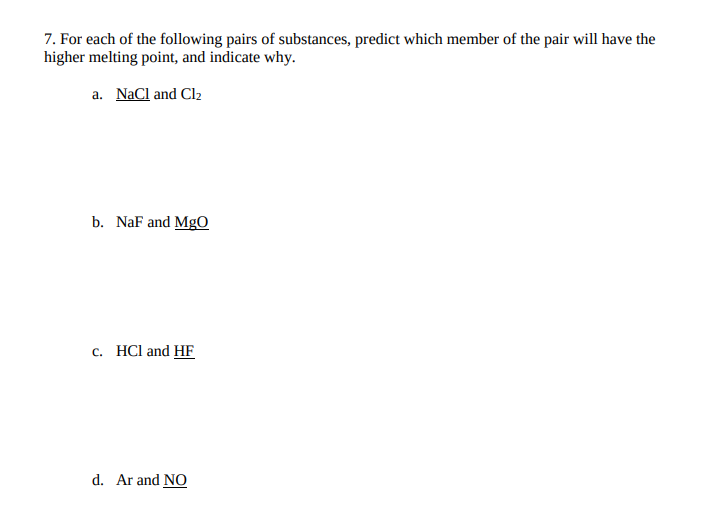
Transcribed Image Text:7. For each of the following pairs of substances, predict which member of the pair will have the
higher melting point, and indicate why.
a. NaCl and Clz
b. NaF and MgO
c. HCl and HF
d. Ar and NO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Define the normal boiling point of water. Why does a sample of boiling water remain at the same temperature until all the water has been boiled? Define the normal freezing point of water. Sketch a representation of a heating/cooling curve for water, marking clearly the normal freezing and boiling points.arrow_forwardA solution of benzoic acid in benzene has a freezing point of 3.1 C and a boiling point of 82.6 C. (The freezing point of pure benzene is 5.50 C, and its boiling point is 80.1 C) The structure of benzoic acid is Benzoic acid, C6H5CO2H What can you conclude about the state of the benzoic acid molecules at the two different temperatures? Recall the discussion of hydrogen bonding in Section 11.3.arrow_forwardWhich should have the higher melting point, MgO or NaCl? (a) MgO (b) Naclarrow_forward
- 58. Define the equilibrium vapor pressure of a substance. How and why does it depend on temperature? What occurs when a liquid boils? 59. What is Raoult's Law? Give an equation and define all terms, including units. When is it applicable and why? Illustrate your answer with a real example.arrow_forwardmatch the following 0.110 m CoI2 A. Highest boiling point 0.180 m NaCH3COO B. Second highest boiling point 0.100 m CuI2 C. Third highest boiling point 0.390 m Sucrose (nonelectrolyte) D. Lowest boiling pointarrow_forwardCalcium sulfate (CaSO4) is insoluble in water. It is calculated that AH for solution is about zero. What is the sign of AS for the solution and why? 1. AS is positive. 2. AS is negative. Because 3. Mixing CaSO4 with water increases the number of possible arrangements. 4. Water molecules have fewer possible arrangements when Ca²+ and SO4²- ions are in solution due to being held tightly by strong ion-dipole interactions. 5. Water molecules exchange easily with those in the solvation shell. O2 and 3 O2 and 5 1 and 4 O2 and 4 0 1 and 3 O 1 and 5arrow_forward
- For the table below, specify the dominant intermolecular force involved for each substance in the space immediately following the substance. Then in the last column, indicate which member of the pair you would expect to have the higher boiling point. Substance #1 Daninant Intermdecular Farce Substance #2 Dominant Intermedecular Fææ Substance with Higher Beling Pint a. HCl(g) b. CH;F CH;OH с. H20 H2S d. SIO2 SO, е. Fe Krarrow_forward28. Refer to the diagram below to determine a. the boiling point of ethanol when it is at normal atmospheric pressure. b. the boiling point of methanol at 96.0 kPa. Vapor Pressure (kPa) c. the atmospheric pressure at which diethyl ether boils at 20 °C. 120 110 100 90+ 80 70+ 60 5 55688 50- 30. 20 10. 0 0 atmospheric pressure (101 kPa) diethyl ether methanol 20 bp 34.6 ethanol bp 64.7 water 40 60 Temperature (°C) bp 78.2¹ bp 100 80 100arrow_forwardArrange the following in terms of increasing boiling point using (<) less than equality.arrow_forward
- 28. Pure water boils at 100°C. What is the new boiling point of water after the addition of 13.12 g aluminum chloride, AIC13, to 615 g water?arrow_forwardEnergy to sublime K(s) = 89.0 kJ/mol Electron affinity of F(g) = -328.0 kJ/mol First ionization energy of K(g) = 425.0 kJ/mol %3D Bond energy of F2(g) = 154.0 kJ/mol AHxn for K(s) + 1/2 F2(g) → KF(s) = -562.6 kJ/mol %3D 1st attempt O See Hint Determine the lattice energy of KF(s), using the data provided. kJ/molarrow_forward1. Adding 1 mol of NaCl to 1 kg of water lowers the vapor pressure of water more than adding 1 mol of C6H1206. Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning