

Reversible reactions always depict specified expression for equilibrium constant (K). This constant carried connectivity among equilibrium concentration of all involved reactant-product.

Since it is given that 2.00 moles of NO and 3.00 moles of Cl2 are present in the container initially which reflects that there is no NOCl amount present in the solution.
Thus the reaction will proceed in the reverse direction as shown below.
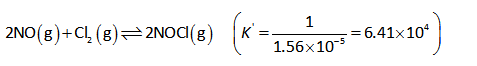
The equilibrium constant expression is shown below.
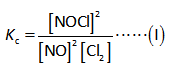
Here,
The equilibrium constant is “Kc”.
The formula for the calculation of the concentration/molarity is shown below.
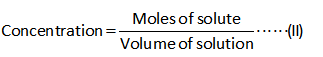
Substitute the known values in the equation (II) to calculate the concentrations.
The concentration of NO:
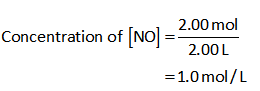
The concentration of Cl2:
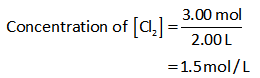
Step by stepSolved in 6 steps with 10 images

- Consider the following reaction: 4NH3(g) + 6NO(g) → 6H₂0(g) + 5N₂(g) If the initial concentrations of NH3(g) and NO(g) are 4.0 M, at equilibrium [NO] = 1.0 M, calculate the equilibrium concentration of NH3(g). A) 3.0 M B) 2.0 M C) 5.0 M D) 2.5 M E) 0.50 Marrow_forward2NO(g) + 2H₂(g) = N₂(g) + 2H₂O(g) The system initially contained 0.2500 M NO, 0.1300 M H2, and 0.2500 M H₂O. At equilibrium, the concentration of NO is 0.1296 M. What is the equilibrium concentration of H₂? [H₂] = [?] x 10¹ M K= [N₂] [H₂O]2 [NO]2[H₂]2 Coefficient (green) Exponent (yellow) Enterarrow_forwardConsider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 104 at a certain temperature. If a solid sample of NH.SH decomposes, what will the equilibrium concentration of NH3 be? NH:SH(s) = NH3(g) + H2S(g) 1 2 3 NEXT > Based on the given values, set up ICE table in order to determine the unknown. NH.SH(s) NH:(g) H2S(g) Initial (M) Change (M) Equilibrium (M) RESET 1.2 x 104 +x +2x -2x 1.2 x 104 + x 1.2 x 104 - x 1.2 x 104 + 2x 1.2 x 104- 2xarrow_forward
- At a certain temperature, the equilibrium constant K for the following reaction is 0.71: N2(g) + O2(g) =2 NO(g) Use this information to complete the following table. Suppose a 43. L reaction vessel is filled with 1.0 mol of NO. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and 02. There will be very little NO. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K =0 2 NO(g) N,(9)+O2(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = 3 N2(9)+30,(9) 6 NO(g)arrow_forwardThe equilibrium constant for the following reaction is 0.18 at a set temperature. Find the equilibrium concentrations if the initial concentration of PCl3 is 0.225 mol/L and the initial concentration of Cl2 is 0.150 mol/L. PCI3(g)+CI2(g)=PCI5(g)arrow_forwardConsider the following equilibrium: 2NOCl (g) <--> 2NO (g) + Cl2 (g) With K = 1.6 x 10-5. 1.00 mole of pure NOCl and 0.964 mole of pure Cl2 are placed in a 1.00 L container. Calculate the equilibrium concentration of Cl2 (g).arrow_forward
- 3. Nitrogen gas and oxygen gas can combine to produce nitrogen monoxide. At 1500 K the equilibrium constant, Ke, is 1.0 x 10 . Suppose a sample of air has [N2] = 0.80 M and [O2] = 0.20 M before any reaction occurs. Calculate the equilibrium concentrations of product and reactants at equilibrium at this temperature.arrow_forwardA student adds 0.600 mol of methane gas, CH4(g) into a 2.00 L container and the methane forms ethyne, C2H2(g) and hydrogen gas, H2(g). At equilibrium, the container was found to contain 0.045 mol/L C2H2.a) Calculate the initial concentration of CH4. Then complete an ICE Table to organize the initial, change and equilibrium concentrations for this equilibrium system. Show any calculations needed. 2CH4(g) ⇄ C2H2(g) + 3H2(g)b) Calculate the equilibrium constant, Keq, for this equilibrium. Show all work.c) What is the equilibrium concentration of H2(g) ?arrow_forwardA mixture of 0.100 mol of SO2 and 0.100 mol of O2 is placed in a reaction container and allowed to react until equilibrium is established. 2 SO2 (g) + O2 (g) -> 2 SO3 At equilibrium, 0.0916 mol of SO3 is present. a. What is the composition of the equilibrium mixture in terms of moles of each substance present? (Hint: Stoichiometry!) b. If the container size is 3.0 L, what is the value of the equilibrium constant?arrow_forward
- 1. Consider the following reaction at 500 K: 2 NO (g) + O2 (g) 2 2 NO2 (g); Kc = 6.9 x 105 This reaction is allowed to reach equilibrium, and then analyzed and found to contain the following concentrations: [O2] = 1.0 x 10-3 M [NO2] = 5.0 x 10-2 M. Determine the equilibrium concentration of NO (g).arrow_forwardConsider the reaction A + B C+D, which has an equilibrium constant, K, equal to 3.4 x 102. If one begins a reaction by placing 0.600 moles of A in a 1.0 L container as well as 0.150 moles of 1. B, what will be the equilibrium concentrations of A, B, C, and D? Write your answers in the spaces provided below. a. [А] b. [B]= [C]= С. d. [D]= Once the reaction in problem 1 reaches equilibrium, some additional B is injected into the flask 2. from an outside source. LeChatelier's principle says the reaction will (circle one): be unchanged shift to the right shift to the left Pyridine is a weak base with a Kb 1.7 x 109. If 0.300 moles of pyridine is added to 1.00 L of 3. water, what will be the equilibrium concentrations of the species below: a. [Pyridine] = b. [Pyridine-H] (the pyridinium ion) [ОН-] 3 С. What is the pH of the solution in problem 4 (above)? .arrow_forwardAt a certain temperature, the reaction 2HF(g) a H2(3) + F2(g) has K = 1.2 x 10-13. Does this reaction proceed far towards completion when equilibrium is reached? If 0.022 mol HF was placed in a 1.00 L container, and permitted to come to equilibrium, what would be the concentration of H2 and F2 in the container? Concentration of F2 at equilibrium = i M Concentration of H2 at equilibrium = i Marrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





