
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
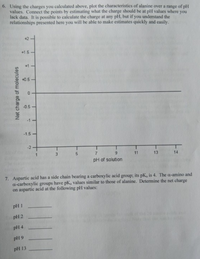
Transcribed Image Text:6. Using the charges you calculated above, plot the characteristics of alanine over a range of pH
values. Connect the points by estimating what the charge should be at pH values where you
lack data. It is possible to calculate the charge at any pH, but if you understand the
relationships presented here you will be able to make estimates quickly and easily.
+2
+1.5
+1 -
+0.5-
-0.5 -
-1-
-1.5 -
7.
6.
11
13
14
pH of solution
7. Aspartic acid has a side chain bearing a carboxylic acid group; its pK, is 4. The a-amino and
a-carboxylic groups have pKa values similar to those of alanine. Determine the net charge
on aspartic acid at the following pH values:
pH 1
pH 2
pH 4
pH 9
pH 13
Net charge of molecules
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- 5. Refer to Model 2 for information relevant to this question. A sample of the peptide Lys-Glu-Ser has a net charge of zero between what two pH values? What is the pl of Lys-Glu-Ser? Model 2: The pl of a peptide is determined by examining the ionizable groups. The protonated and unprotonated forms of each ionizable group are in equilibrium. Consider the peptide Lys-Glu-Ser shown below at pH 7.2. The complete structure is on on the left and a stylized structure with just the ionizable groups is on the right. While the N-terminal is depicted as protonated, a sample of Lys-Glu-Ser is composed of a population of molecules and within that population some molecules contain a non-protonated N-terminal group at pH 7.2. may H3N* COO- H3N-CH-CIN-CH C N-CH C- H. pK 3.5 OH H. pK= 8.5 COO- CH2 CH2 CH2 NH, pK 10.0 pK 4.2 CH2 CH2 OH CH2 c=0arrow_forwardWhich of the amino acid side-chains would you expect to be protonated (i.e. has not lost its proton) under neutral pH conditions? Include reasons supporting your prediction.arrow_forwardConsider this structure. NH₂ N N O P O N N Part 1 of 3 HO Identify the monosaccharide. Select the single best answer. D-ribose D-2-deoxyribose Part: 1/3 Part 2 of 3 Identify the base. Select the single best answer. Othymine O guanine ○ cytosine adenine uracilarrow_forward
- Can you please explain how to solve this problem?arrow_forwardWould each of the following ions of serine exist at a pH above, below, or at its pI? Drag the appropriate items to their respective bins. Above pl H₂N-CH-COO CH₂OH H₂N-CH-COOH CH₂OH Below pl H₂N-CH-COO CH₂OH At its pl Reset Helparrow_forward2) Consider the following disaccharide which was formed from the breakdown of a polysaccharide found in plant material. ОН Glycosidic linkage: H H OH H H НО H H. OH H. ОН OH H НО H ОН a. Label the glycosidic linkage above in the following format: a/B(# → #) b. Would you expect this disaccharide to have been formed from amylose or amylopectin? c. Describe at least two chemical tests that would give insight into the structure of this disaccharide, assuming you've isolated it and wanted to elucidate the structure from scratch. Include detail on not only the tests you would use but what results you would expect to get given the structure above. Assume you can analyze the structure of any monosaccharide you get from your chemical tests.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education