
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
7-9

Transcribed Image Text:9) What would be the chemical shift and spin-splitting ('H NMR) for hydrogens circled in the
following compound?
A
(CH3
(a) A = 5.5 ppm, quartet; B = 2.2 ppm, singlet
(c) A = 4.5 ppm, quintet; B = 1.2 ppm, doublet (d) A = 5.5 ppm, quartet; B =2.2 ppm, double
%3D
(b) A = 4.5, quartet; B = 1.2 ppm, singlet
10) Which of the following compounds would contain characteristic IR stretches at 3300 and
2170 cm-1?
A) CH3CH2CHO
B) CH3CH=CHCH2OH
C) (CH3)2CHC=N (D) CH3CH2CH2C=CH
E) CH3C=CCH2CH3
aing
11) Which compound would be expected to show intense IR absorption at 1660 cm-1?
A) CH3CH2OCH2CH3
C) CH3CO2CH2CH3
yi
B) CH3CH2C=N
) CH3CH2CONH2
E) CH3COCH2CH3
12) What major functional groups are represented in the following IR spectrum?
(6) 1
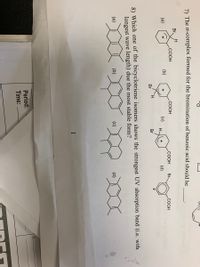
Transcribed Image Text:7) The o-complex formed for the bromination of benzoic acid should be
Br.
.COOH
.COOH
.COOH
Br
СООН
(a)
(b)
(c)
H-
+
(d)
H.
Br
Br
8) Which one of the bicyclotriene isomers shows the strongest UV absorption band (i.e. with
longest wave length) due the most stable form?
(a)
(b)
(c)
(d)
1
Period:
Time:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the VSEPR model to predict the bond angles about each highlighted atom.arrow_forwardCan u box ur final answer plz ??arrow_forwardPQ-24. On which of the numbered atoms in this structure will the negative charge be delocalized? (A) 2,4,6 PO 25 WE (B) 2, 4, 6, 8 (C) 1,3,5 3 2 4 6 CH₂ 7 8 (D) 1,3,5,9arrow_forward
- 5-57 You know the mechanism of HBr addition to alkenes, and you know the effects of various substituent groups on aromatic substitution. Use this knowledge to predict which of the following two alkenes reacts faster with HBr. Explain your answer by drawing resonance structures of the carbo- cation intermediates. CH=CH2 .CH=CH2 and CH30 O2Narrow_forwardQUESTION 4 (-)-5-Methyl-2-(1-methylethyl)cyclohexanol, also known as menthol, is used to treat minor throat irritation and can be isolated from mint plants. The specific rotation of menthol at 20 °C is -48° mL g1 dm-1 and its melting point is 43 °C. Use the Lewis structure shown below to answer problems 4-7. ta HO Menthol can also be called (1R,2S,5R)-5-methyl-2-(1-methylethyl)cyclohexanol based on the absolute configuration of its 3 stereocenters. Which of the following stereoisomers is the enantiomer(s) of menthol? Choose all that apply. O A. (1R,2S,59)-5-methyl-2-(1-methylethyl)cyclohexanol O B. (1S,2S,5S)-5-methyl-2-(1-methylethyl)cyclohexanol O'C. (1R,2R,5R)-5-methyl-2-(1-methylethyl)cyclohexanol O D. (1S,2R,5S)-5-methyl-2-(1-methylethyl)cyclohexanolarrow_forwardProblem 7arrow_forward
- OH H,SO, 7.arrow_forward8:23 PM Wed Feb 2 * 41% Question 10 of 30 O Done Draw the structural condensed form of 4-methyl-2-pentene. Atoms, Bonds and Rings Charges Draw or tap a new bond to see smart suggestions. Undo Remove Resetarrow_forward4- What is the product formed in the following reaction? 요 + NaOH Heat a) ii-iii b) only ii c) only iii d) i-ii e) No reaction i Цо ii iii ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY