
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
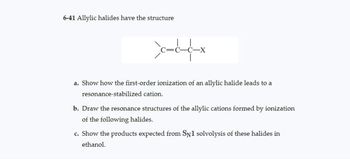
Transcribed Image Text:6-41 Allylic halides have the structure
cccx
a. Show how the first-order ionization of an allylic halide leads to a
resonance-stabilized cation.
b. Draw the resonance structures of the allylic cations formed by ionization
of the following halides.
c. Show the products expected from SN1 solvolysis of these halides in
ethanol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 6arrow_forward5. Use curved arrows to show the mechanism of the following acid-base reaction: "H ¡N-H H y N-H Harrow_forwardReaction B H Br EtOH H Br Figure 2 - Reaction Scheme B. In this method, stilbene is brominated using pyridinium bromide. Ethanol is used as the solvent. 40.0 mL of ethanol and 2.00 g of (E)-stilbene were placed in a 125 mL Erlenmeyer flask. The mixture was heated on a hot plate to dissolve the stilbene, after which 4.00 g of pyridinium tribromide was added to the mixture. After heating while stirring for 5 minutes, the Erlenmeyer flask was removed from heat, and the dibromide product was allowed to crystallize as the flask cooled to room temperature. Next, the mixture was chilled in an ice bath, and the product was collected by vacuum filtration, washing the crystals with 5.00 mL of ice-cold methanol. The product was allowed to air-dry, and obtained as white, needle-like crystals. Table 3: Usage and Waste Data for Scheme B Properties Amounts Used Excess / Waste Moles Compound MW (g/mol) Mass (kg) (mol) Stilbene 11.1 180.25 2.00 kg Ethanol Pyridinium 4g tribromide Methanol Product -H…arrow_forward
- Cycloaddition reactions are useful tools for building rings by using electrons to form o bonds between atoms. For the products below, identify a reasonable set of starting materials to make each product. NH₂ O g O Which of the two sets of starting materials you proposed above do you think would react more quickly? Think about what makes a cycloaddition fast or slow and justify your answer.arrow_forward4. Consider the reactants below. Answer the following questions about the reaction mechanism and products. Br x a. What is the degree of substitution of the alkyl halide? b. What type of base and nucleophile is the water molecule? A H₂O c. What type of mechanism is favored in the reaction of tertiary halides and weak bases/nucleophiles with heat applied? d. Draw the structure of the product for the reaction e. Provide an arrow-pushing mechanism for the formation of the product.arrow_forwardWhich compound (structure with letter) best fits the product of the following reaction? ∞ = DMS H Br B 8888 BSD OH Br Br 8 $$$ from H Br E Br ? L Br OH Br M Br OHarrow_forward
- 9. Rank the following nucleophiles, in a solution of DMF, in order of weakest to strongest. НО CI Strong Br Slow II II IVarrow_forwardProvide the major organic products for the followingarrow_forward6:39 ← Question 7 of 22 Draw the major product of this reaction. Ignore inorganic byproducts. 1. LDA, -78 °C Submit 2. PhCH₂Br (1 equiv)arrow_forward
- 3. Predict the products in the following reactions. ai 요 Hoi HO Br KMnO4, NaOH 0 °C 1. KMnO4, NaOH, A 2. dil. H₂SO4¹ H₂O 1. KMnO4, NaOH, A 2. dil. H₂SO4, H₂O Zn(Hg) HCI H NaBH₂CN, EtOH, pH 5 PBr3arrow_forwarddestion 1 What product is expected from the reaction below? 1. Mg /dry ether 3. H*/H;0 МаBr OH 2. 3 A. 1 O B. 2 OD. 3 OE. 4arrow_forwardI need help with thisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY