
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:-7
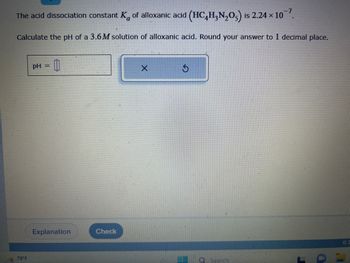
The acid dissociation constant K of alloxanic acid (HC4H₂N₂O) is 2.24 × 10¯7.
Calculate the pH of a 3.6M solution of alloxanic acid. Round your answer to 1 decimal place.
78°F
pH =
0
Explanation
Check
X
Ś
O
Search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pure liquid ammonia ionizes in a manner similar to that of water. (a) Write the equilibrium for the autoionization of liquid ammonia. (b) Identify the conjugate acid form and the base form of the solvent. (c) Is NaNH2 an acid or a base in this solvent? (d) Is ammonium bromide an acid or a base in this solvent?arrow_forwardTo measure the relative strengths of bases stronger than OH, it is necessary to choose a solvent that is a weaker acid than water. One such solvent is liquid ammonia. (a) Write a chemical equation for the autoionization of ammonia. (b) What is the strongest acid and base that can exist in liquid ammonia? (c) Will a solution of HCI in liquid ammonia be a strong electrical conductor, a weak conductor, or a nonconductor? (d) Oxide ion (O2) is a stronger base than the amide ion (NH2). Write an equation for the reaction of O2 with NH3 in liquid ammonia. Will the equilibrium favor products or reactants?arrow_forwardUsing the diagrams shown in Problem 10-37, which of the four acids is the weakest acid?arrow_forward
- Ethanol (ethyl alcohol), CH3CH2OH, can act as a BrnstedLowry acid. Write the chemical equation for the reaction of ethanol as an acid with hydroxide ion, OH. Ethanol can also react as a BrnstedLowry base. Write the chemical equation for the reaction of ethanol as a base with hydronium ion, H3O+. Explain how you arrived at these chemical equations. Both of these reactions can also be considered Lewis acid base reactions. Explain this.arrow_forwardWhich acid has the strongest conjugate base? (a) HNO2 (b) C6H5CO2H (c) HCN (d) HClarrow_forwardIn each of the following acid-base reactions, identify the Brnsted acid and base on the left and their conjugate partners on the right. (a) HCO2H(aq) + H2O() HCO2(aq) + H3O+(aq) (b) NH3(aq) + H2S(aq) NH4+(aq) + HS(aq) (c) HSO4(aq) + OH(aq) SO42(aq) + H2O+()arrow_forward
- Which of the following substances are acids in terms of the Arrhenius concept? Which are bases? Show the acid or base character by using chemical equations. a P4O10 b Na2O c N2H4 d H2Tearrow_forwardFor oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forwardWhich of the terms weak, strong, monoprotic, diprotic, and triprotic characterize(s) each of the following acids? More than one term may apply in a given situation. a. HC3H3O3 b. HCN c. H2SO4 d. H2SO3arrow_forward
- Consider the following four solutions: (1) apple juice, pH 3.8, (2) pickle juice, pH 3.5, (3) carbonated beverage, pH 3.0, and (4) drinking water, pH 7.2. a. Which solution has the highest [H3O+]? b. Which solution has the highest [OH]? c. List the solutions in order of increasing acidity. d. List the solutions in order of decreasing basicity.arrow_forwardWhich of the terms weak, strong, monoprotic, diprotic, and triprotic characterize(s) each of the following acids? More than one term may apply in a given situation. a. H3PO4 b. H3PO3 c. HBr d. HC2H3O2arrow_forwardIn each of the following acid-base reactions, identify the Brnsted acid and base on the left and their conjugate partners on the right. (a) C2H5N(aq) + CH3CO2H(aq) C5H5NH+(aq) + CH3CO2(aq) (b) N2H4(aq) + HSO4(aq) N2H5+(aq) + SO42(aq) (c) [Al(H2O)6]3+ (aq) + OH(aq) [Al(H2O)5OH]2+ (aq) + H2O+()arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning