Question
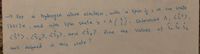
Transcribed Image Text:-7 For
hydrogen atom electron, with
Spin , in the state
a
-A(), Determine A, <î?>,
of Sx, Sy,S2
13217水
and with spin State x
く>,くら、?,くら,>, and <sを7. Ane
Values
well defined
in this stae ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions