
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
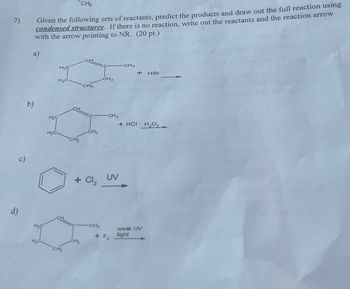
Transcribed Image Text:7)
CH2
Given the following sets of reactants, predict the products and draw out the full reaction using
condensed structures. If there is no reaction, write out the reactants and the reaction arrow
with the arrow pointing to NR. (20 pt.)
a)
CH
H2C
H₂C.
CH2
b)
H2C
H₂C
CH
CH2
CH2
d)
H₂C
H₂C.
CH 2
CH3
+ HBr
CH3
+ HCI H₂O
+ Cl₂
UV
CH3
weak UV
+ F2
light
CH2
CH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- ✓ CH3I, NH3 N :I: O Select to Add Arrows :I: CH3 O H H CH3I, NH3 CH3I, NH3 > H :I: O H Select to Add Arrows CH3I, NH3 H3C H H :I: O Select to Add Arrows HI I I I 1 I I I I I Problem 37 of 40 Please select a drawing or reagent from the question area Submitarrow_forward?arrow_forward3. Name all the functional group/s in the following organic compound. This is the chemical compound 'capsaicin' - the source of the heat in hot chili peppers. HHOH H HHH H -C-C-C-C-C%3DC-C-CH3 T T T T H HHH -C-N-C-C- H CH3 a. b. H. H. C- H3C-O C-C- C-C C. d.arrow_forward
- 1 M B W Chapter... C G Provide the systematic (IUPAC) name for the following hydrocarbons. (a) (b) H3C- Marvin JS H₂ P1₂₂ HC H₂C H₂ H₂C-CH₂ H₂ H₂ CH3 H₂ Write the structural formulas for the following hydrocarbons. (c) 2,2-dimethylbutane G ↑ webassign.net Help C MacBook Airarrow_forwardO HYDROCARBONS Predicting the reactants or products of alkene hydrogenation ive one possibility for the mystery reactant R in this organic reaction: R + H₂ Pd CH3 CH2 - CH2 - CH3 - Specifically, in the drawing area below draw the condensed structure of what R might be. There Note: keep in mind that the equation above states R and H₂ are present in a 1:1 mole ratio. Click anywhere to draw the first YE C Xarrow_forwardIn problem 1.30 letter f my structure is different than the one in the book . I don’t know whyarrow_forward
- HH + H H-C- H 1. 2₁ 3₁ H Organic Compounds H 'S H-0-0-0-0-0-H -H H HH H-C-C=C-Br Answer Classification 4. H-C C-O-H Unsaturated compound Alcohol Ester Aromatic compound 6. Match each of the diagrams above with its classification below. CH, CH₂CH₂CH,arrow_forward1.1 Introduction to Organic Chemistry Which of the molecules below is NOT organic? O H-N-H H. H. H-C-OH H. H. H. H-C-O- C-H H. H-C-H H. Save for Laterarrow_forwardprepaer the follwing compoundsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY