
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
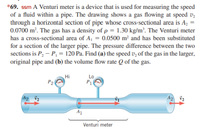
Transcribed Image Text:*69. ssm A Venturi meter is a device that is used for measuring the speed
of a fluid within a pipe. The drawing shows a gas flowing at speed v,
through a horizontal section of pipe whose cross-sectional area is Az =
0.0700 m². The gas has a density of p = 1.30 kg/m². The Venturi meter
has a cross-sectional area of A, = 0.0500 m² and has been substituted
for a section of the larger pipe. The pressure difference between the two
sections is P, – P, = 120 Pa. Find (a) the speed v, of the gas in the larger,
original pipe and (b) the volume flow rate Q of the gas.
Hi
Lo
P1
A2 v2
A2 v2
A1
Venturi meter
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the balloon less dense then the surrounding air, the balloon is able to lift objects many times its own weight. A large hot air balloon has a maximum balloon volume of 2090 m3 a. What is the density of air inside the balloon, in terms of the pressure P, temperature T, molar mass M, and the gas constant R? b. How much mass can this balloon lift (in addition to the mass of the gas inside) in terms the balloon volume Vb, the atmosphere air density ρa, the density of the air in the balloon ρg, and the gravitational acceleration g? c. If the air temperature in the balloon is 54 °C, how much additional mass, in kilograms, can the balloon lift? Assume the molar mass of air is 28.97 g/mol, the air density is 1.20 kg/m3, and the air pressure is 1 atm.arrow_forwardA sphygmomanometer is a device used to measure blood pressure, typically consisting of an inflatable cuff and a manometer used to measure air pressure in the cuff. In a mercury sphygmomanometer, blood pressure is related to the difference in helghts between two columns of mercury. The mercury sphygmomanometer shown in the figure below contains air at the cuff pressure P. Po The difference in mercury heights between the left tube and the right tube is h= 109 mmtg - 0.109 m, a normal systolic reading. What is the gauge systolic blood pressure Poauge in pascals? The density of mercury is p 13.6 x 10 kg/m and the ambient pressure is Po1.01 x 10 Pa. HINT Paarrow_forwardA PROBLEMS 6.63, 6.64 6.64 This "double" nozzle discharges water (at 10°C) into the atmosphere at a rate of 0.50 m /s. If the nozzle is lying in a horizontal plane, what x-component of force acting through the flange bolts is required to hold the nozzle in place? Note: As- sume irrotational flow, and assume the water speed in each jet to be the same. Jet A is 10 cm in diameter, jet B is 12 cm in diame- ter, and the pipe is 30 cm in diameter.arrow_forward
- A basketball is pressurized to a gauge pressure of PG = 55 kPa when at the surface of a swimming pool. (Patm = 101 kPa). The ball is then submerged in the pool of water which has a density ρ = 1000 kg/m3. Assume the ball does not change in mass, temperature, or volume as it is submerged. Calculate the absolute pressure inside the basketball in kPa when it is at the surface. Write an equation for the pressure difference ΔP between the inside and outside of the ball when it is submerged a distance y below the surface of the water. Solve the pressure equation for the depth (in meters) at which the pressure difference between the inside and outside of the ball will become zero. At this depth the pressure inside the basketball is the same as the pressure outside the ball.arrow_forward19. The radius of the bronchial tube is decreased by a factor of 4. The velocity of gas through it initially is 1 m/s. What is the velocity in the section of bronchial tube with decreased radius in m/s?arrow_forwardA balloon is released from a tall building. The total mass of the balloon including the enclosed gas is 2.0 kg. Its volume is 5.0 m?. The density of air is 1.3 kg/m. 12. What is the average density of the balloon? A) 0.2 kg/m3 B) 0.4 kg/m3 C) 0.8 kg/m D) 1.0 kg/m E) 1.2 kg/m3 13. Will the balloon rise, fall, or remain stationary, and why? A) The balloon will fall because its density is greater than that of air. B) The balloon will remain stationary because its density is less than that of air. c) The balloon will rise because the upward buoyant force is greater than its weight. D) The balloon will fall because the upward buoyant force is less than its weight. E) The balloon will fall because the downward buoyant force is greater than the upward buoyant force.arrow_forward
- A vertical, frictionless piston-cylinder device contains a gas at 600 kPa absolute. The atmospheric pressure outside is 100 kPa absolute. The piston has a diameter of 50 mm. a) Determine the mass of the piston. b) If the mass of the piston is now halved ---- what would be the gauge pressure of the gas?arrow_forwardA cylinder containing ideal gas is sealed by a piston that is above the gas. The piston is a cylindrical object, with a weight of 22.0 N, which can slide up or down in the cylinder without friction. The inner radius of the cylinder, and the radius of the piston, is 8.00 cm. The top of the piston is exposed to the atmosphere, and the atmospheric pressure is 101.3 kPa. The cylinder has a height of 30.0 cm, and, when the temperature of the gas is 20°C, the bottom of the piston is 11.0 cm above the bottom of the cylinder. (A) Find the number of moles of ideal gas in the cylinder. (B) Heat is added, gradually raising the temperature of the gas to 160°C. Calculate the distance between the bottom of the cylinder and the bottom of the piston when the piston comes to its new equilibrium position.arrow_forwardWhat is the cause of air pressure? Why does the pressure a fluid exerts on the walls of its container decrease when the fluid starts to flow through the container (e.g. a piece of tubing)? Why is it impossible to pull a fluid? What does it mean to "entrain a fluid" into a flow? When the gauge on a cylinder of gas reads zero, is the cylinder actually empty? Explain.arrow_forward
- Tire gauges for air pressure, as well as most other gauges used in an industrial environment take into account the pressure due to the atmosphere of earth. That's why your car gauge reads 0 before you put it on your tire to check your pressure. That is called gauge pressure. The real pressure within a tire or other object contains pressurized stuff would be a combination of what the gauge reads as well at the atmospheric pressure. If a gauge on a tire reads 40.16 psi, what is the real pressure in the tire in pascals. The atmospheric pressure is 1.01x10^5 Pa.arrow_forwardAnswer question a b and c, if possiblearrow_forwardBarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON