
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
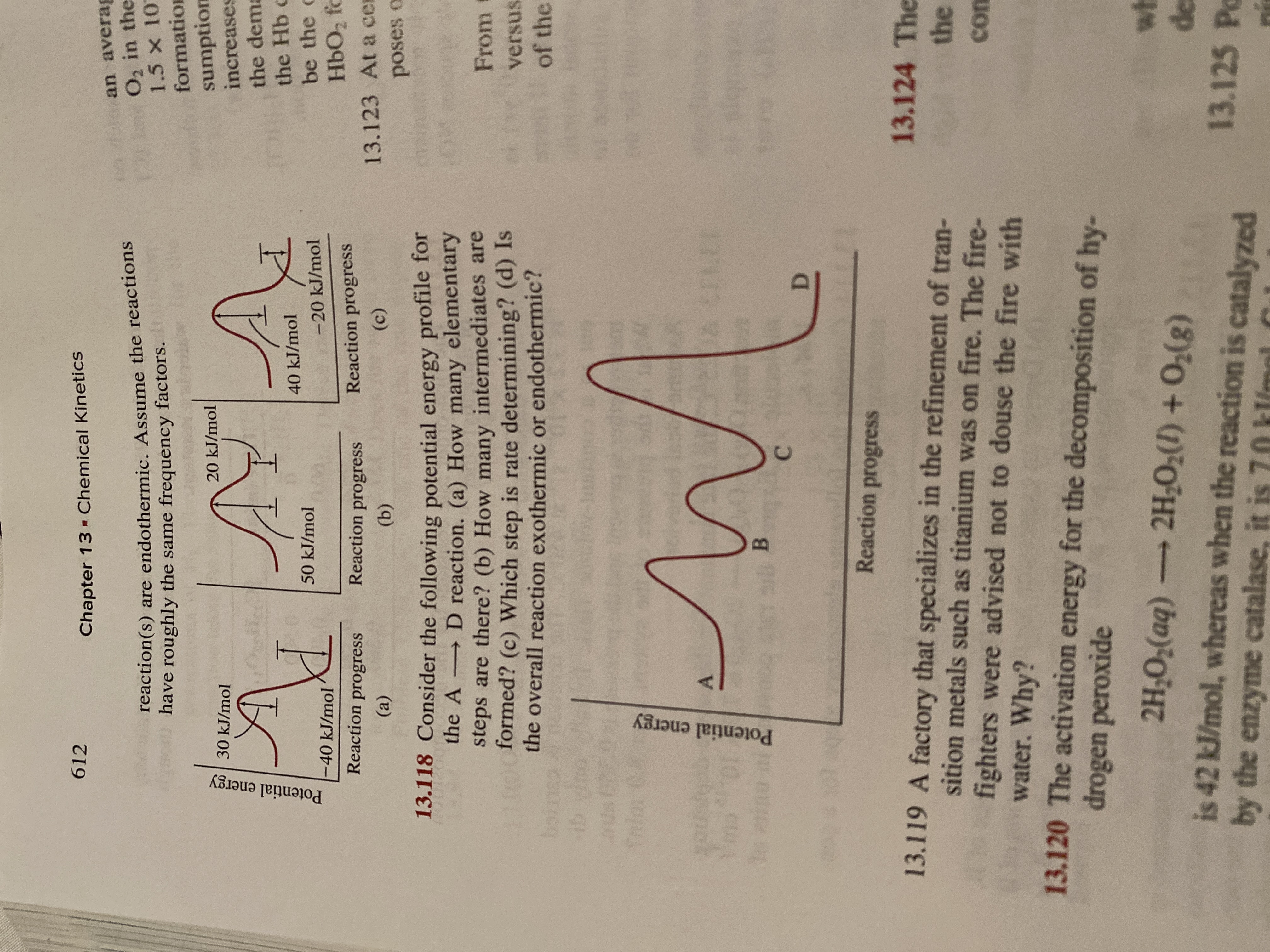
My question is 13.118
I have an idea of how to do it but i am a bit confused as to what is what
Transcribed Image Text:612
Chapter 13 - Chemical Kinetics
an averag
reaction(s) are endothermic. Assume the reactions
have roughly the same frequency factors.
Prin O, in the
1.5 x 10
formation
sumption
increases
20 kJ/mol
30 kJ/mol
the dema
the Hb c
be the c
40 kJ/mol
50 kJ/mol
-40 kJ/mol
-20 kJ/mol
HbO2 fc
Reaction progress
Reaction progress
Reaction progress
13.123 At a cer
(a)
(b)
(c)
poses o
13.118 Consider the following potential energy profile for
→ D reaction. (a) How many elementary
steps are there? (b) How many intermediates are
formed? (c) Which step is rate determining? (d) Is
the overall reaction exothermic or endothermic?
From
versus
of the
boins
ib vi
A CELER
Reaction progress
13.124 The
13.119 A factory that specializes in the refinement of tran-
sition metals such as titanium was on fire. The fire-
fighters were advised not to douse the fire with
water. Why?
the
con
13.120 The activation energy for the decomposition of hy-
drogen peroxide
wh
de
13.125 Po
2H;O;(aq) -
2H-O2(1) + O2(g)
is 42 kJ/mol, whereas when the reaction is catalyzed
by the enzyme catalase, it is 7.0 kl/mol
Potential energy
Potential energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Define and give example for each of the chemistry terms: Hygroscopic substance: Pyrolysis: Ignition: Supersaturated solution: Masking agent: Thermogravimetric: Analysis: Nucleation:arrow_forwardI need the answerarrow_forwardWhen you open a bottle of soda, bubbles rapidly form and gas fizzes out. What is the best explanation of this? Group of answer choices (a) Gas that was trapped at the top of the bottle between the cap and the surface of the liquid escapes immediately and 'draws' liquid soda as it escapes, creating the illusion of bubbling. (b) The act of opening the bottle 'dislodges' tiny, nearly invisible gas bubbles that were clinging to the side of the container. They quickly condense into larger bubbles and escape. (c) Warm air from outside the bottle heats the soda, decreasing the solubility of the CO2 in the soda and causing it to bubble out. (d) When the bottle is opened, the pressure of CO2 gas above the liquid rapidly drops, which significantly decreases the solubility of CO2 in the soda, causing it to bubble out. (e) H2CO3 in the soda reacts with water vapor in the air, forming CO2 that quickly bubbles out.arrow_forward
- Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?arrow_forwardBoiling point of distilled water 97℃ Boiling point of solution 99℃ Mass of flask 118.2g Mass of distilled water w/ flask 209.2 Mass of solute 9.3g what is the difference of temperarure of the two boiling points?arrow_forwardQ2) 90 proof bourbon is 45% ethanol (CH,CH2OH). Determine each of the following for this material. You may need to know that the density of this bourbon is 0.9227 g/ml (1), pure ethanol is 0.7893 g/mL (2), and water (assume the rest is just water) is 1.00 g/mL. a) What is the mole fraction ethanol? b) What is the molarity of the ethanol? c) What is the molality of the ethanol? Q3) Exercise #23 from your text has CO2 in a drink at 0.10 M concentration but only under high pressure. When you open a can, the pressure drops to 1.0 atm. How much (moles) CO, is released from the can when it is opened? [Note: think about how #23 was solved and then do that again for the changed question here. The difference should give you your answer..] Q4) Assuming ideal solution behavior, what is the boiling point of a solution of the sugar solution in Q1 above? [You will need table 11.2 from the text for this)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY