
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please I need part B and C
Transcribed Image Text:s://openvellum.ecollege.com/course.html?courseld=165915728&OpenVellumHMAC=6d7471c-
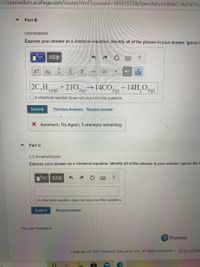
Part B
cycloheptane
Express your answer as a chemical equation. Identify all of the phases in your answer. Ignore
ΑΣφ
?
a
a
2C,H14()
+210
→14CO,
+ 14H,O2(g)
2(g)
2(g)
DA chemical reaction does not occur for this question.
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Part C
2,3-dimethylbutane
Express your answer as a chemical equation. Identify all of the phases in your answer. Ignore the h
ΑΣΦ
OA chemical reaction does not occur for this question.
Submit
Request Answer
Provide Feedback
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. Terms of Use
Expert Solution
arrow_forward
Step 1
Hi, as you have not provided what reaction you want us to write for these compounds. So, considering the examples from Google we are assuming that you are asking for combustion reaction of compounds.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H3C CN 1) i-Bu₂AIH CH3 Ph 2) H3O+arrow_forwardi need the answer quicklyarrow_forwardWhat is the purpose of including a melting point in a procedure? A) As a method of purification - We can purify a substance. B) As a method of encouraging synthesis - Reagents are always more reactive in the liquid state. C) As a method of encouraging a substance to dissolve - Free molecules in the liquid state can make interactions and dissolve in the solvent easier. D) As a method of characterization We can identify an unknown substance. -- O A ODarrow_forward
- *.4 We CH3 H3C- CH3 -CH3 H3Carrow_forwardPart D One of these liquids is used as a "blowing agent" in the manufacture of polystyrene foam because it is so volatile. Which liquid would you expect to be used as a blowing agent? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help Pentane is used as the "blowing agent" because it has a boiling point and is Ethylene glycol volatile low high Submit Request Answer < Return to Assignment Provide Feedback 6:16 PM O 4) 12/12/2021 amazon B. Mastering. Spotify Free 65°Farrow_forwardProblem 35 of 85 Submit Draw the missing organic structures or select the missing reagents in the following multistep synthesis. Ignore any inorganic byproducts formed. 1. NaOCH2CH3 2. CH3CH2Br (1 equiv) Select to Draw H3O+ heatarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY